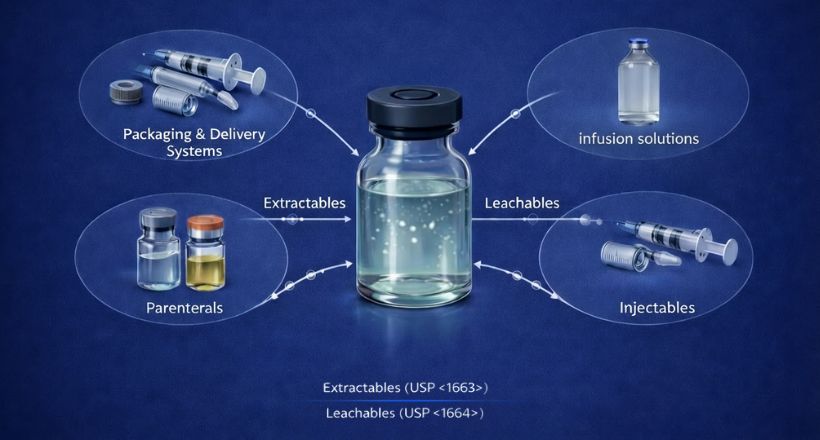
As regulatory expectations for Extractables & Leachables (E&L) continue to evolve, pharmaceutical and biopharmaceutical manufacturers are under increasing pressure to demonstrate accurate, reproducible, and scientifically justified quantification of leachable compounds. Among the most closely scrutinized substances are rubber oligomers originating from butyl and halobutyl elastomers used in container closure systems such as vial stoppers, syringe plungers, and seals.
For parenteral drug products and therapeutic proteins, elastomer-derived leachables are no longer viewed solely as chemical impurities- they are increasingly evaluated as potential contributors to toxicity and immunogenicity risk, making robust E&L programs essential for regulatory compliance and patient safety.
What Are Rubber Oligomers?
Rubber oligomers are low-molecular-weight polymer fragments that originate from elastomeric materials during polymerization, curing, and compounding processes. These species are inherent to elastomer formulations and are commonly associated with butyl and halobutyl rubbers used in pharmaceutical packaging and drug-delivery components.
Due to their relatively small molecular size and increased mobility compared to high-molecular-weight polymers, rubber oligomers can migrate from elastomeric components into drug products over time, particularly under conditions of:
- Long-term storage
- Elevated temperature
- Direct contact with liquid formulations
As a result, rubber oligomers are recognized as an important class of extractables and leachables in pharmaceutical and biopharmaceutical products.
Why Rubber Oligomers Matter for Drug Product Safety
From a product-quality and patient-safety perspective, rubber oligomers are significant because they may:
- Leach into drug products during storage or use
- Interact chemically with active pharmaceutical ingredients, especially sensitive biologics
- Contribute to toxicological or immunogenicity risk at sufficient exposure levels
Depending on the elastomer formulation, rubber oligomers may include saturated, unsaturated, or halogenated species. Brominated rubber oligomers (e.g., C₁₃H₂₃Br) are representative examples associated with halobutyl elastomers and have been cited in regulatory discussions on leachables of potential concern.
Regulatory Perspective: Rubber Oligomers and Immunogenicity Risk
The FDA’s Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products (2014) explicitly recognizes leachables from container closure systems as potential contributors to enhanced immunogenicity and/or toxicity in biological drug products.
Elastomer-derived compounds, including rubber oligomers such as C₁₃H₂₃Br, are representative examples of leachables that may contribute to these effects. For biopharmaceutical products—particularly injectables and long-acting formulations—this elevates rubber oligomers from a packaging concern to a critical component of immunogenicity risk assessment.
Alignment with USP <1663> and <1664>
FDA expectations for biologics intersect closely with pharmacopeial guidance on E&L testing. USP <1663> (Assessment of Extractables) and USP <1664> (Assessment of Drug Product Leachables) emphasize:
- Comprehensive identification of potential extractables
- Quantitative evaluation of actual leachables in drug products
- Exposure-based risk assessment supported by scientifically sound data
For elastomer-derived compounds, this means that tentative identification is no longer sufficient—regulators increasingly expect defensible quantitative data that can be used to assess patient risk.
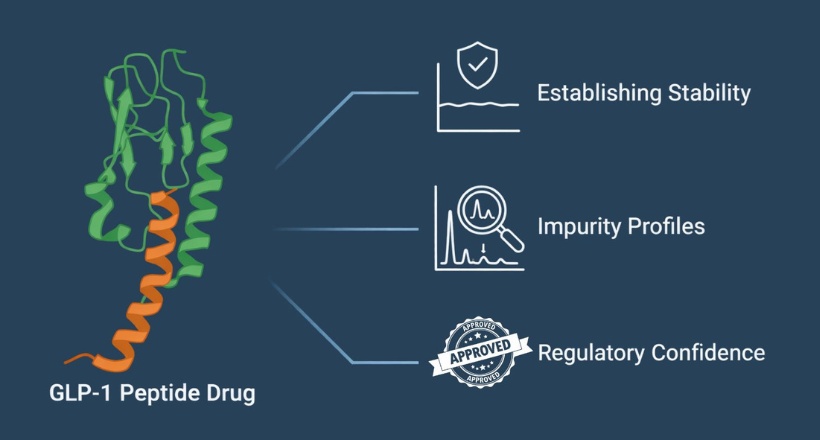
The Quantification Challenge: Why Rubber Oligomer Standards Are Essential
Analytical techniques such as GC-MS and GC-MS/MS are well suited for detecting rubber oligomers at trace levels. However, accurate quantification requires compound-specific certified standards.
Without authentic rubber oligomer standards, laboratories often rely on:
- Surrogate compounds
- Estimated response factors
- Semi-quantitative approaches
These methods introduce uncertainty into:
- Patient exposure calculations
- Toxicological and immunogenicity risk assessments
- Regulatory submissions and justifications
As regulatory scrutiny increases, robust, compound-matched quantification is essential
Daicel Pharma: Integrated Rubber Oligomer E&L Solutions
Daicel Pharma supports pharmaceutical and biopharmaceutical manufacturers through a comprehensive, integrated approach to rubber oligomer assessment.
Rubber Oligomer Certified Standards
- Developed multiple Rubber Oligomer Standards
- Structurally characterized and Certified
- Designed for accurate GC-MS / GC-MS/MS quantification
E&L Study Services Using Rubber Oligomer Standards
- Extractables and leachables studies that directly incorporate compound-specific standards
- Improved accuracy, reproducibility, and inter-laboratory consistency
- Data packages aligned with USP <1663>/<1664> and FDA expectations
By integrating authentic standards directly into E&L study execution, Daicel helps reduce analytical uncertainty and strengthen regulatory confidence.
Why Choose Daicel Pharma?
- Deep expertise in rubber oligomer chemistry
- Purpose-built general standards—not general substitutes
- Integrated analytical and study execution capabilities
- Focus on quantification, reproducibility, and regulatory confidence
Talk to Our E&L Experts
Whether you need rubber oligomer certified standards or comprehensive E&L studies, Daicel Pharma can support your program.
👉 Contact us today to discuss your rubber oligomer E&L study needs

Write a comment
Your email address will not be published. All fields are required