Lovastatin
General Information
Lovastatin Impurities and Lovastatin
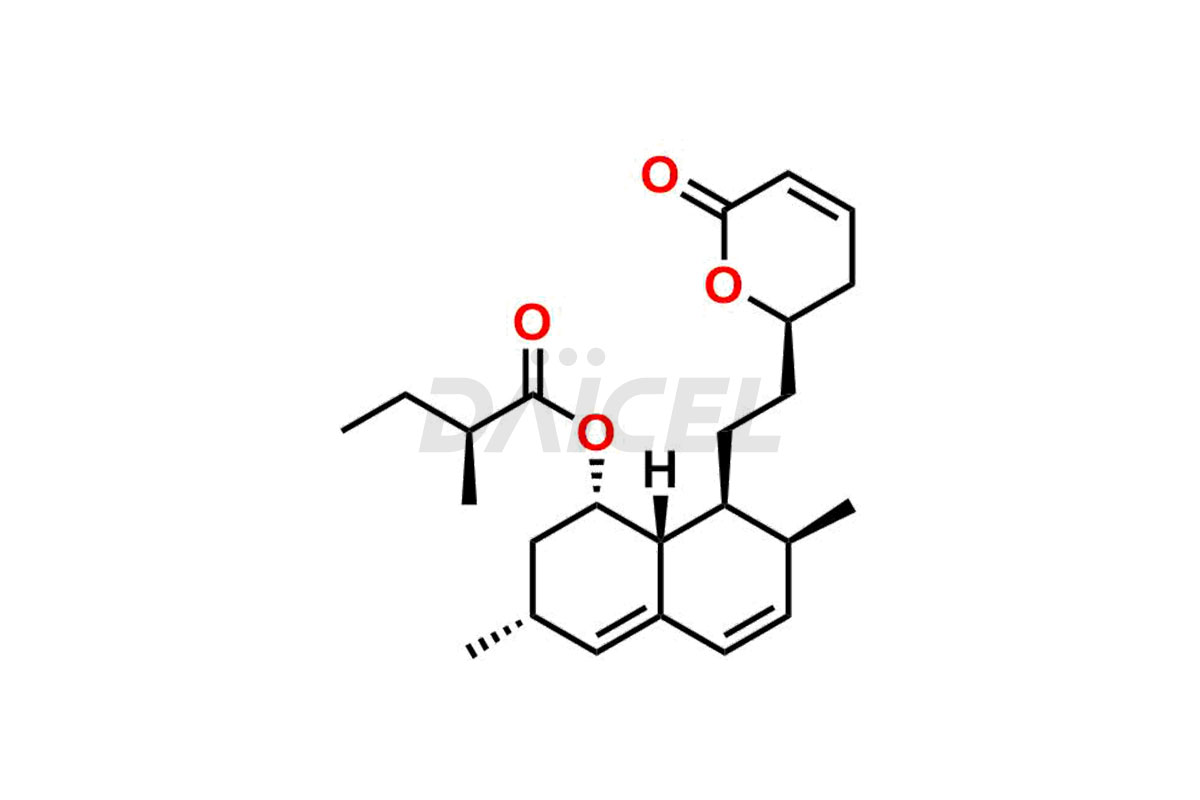
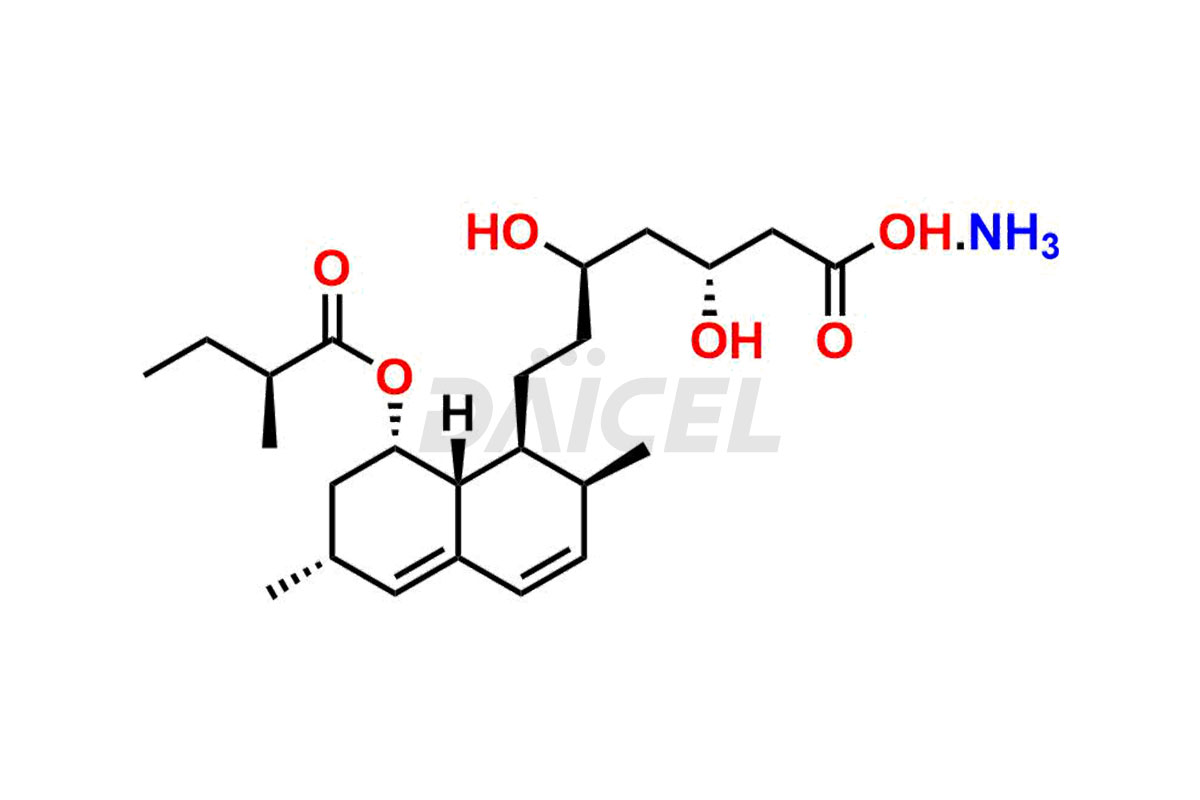
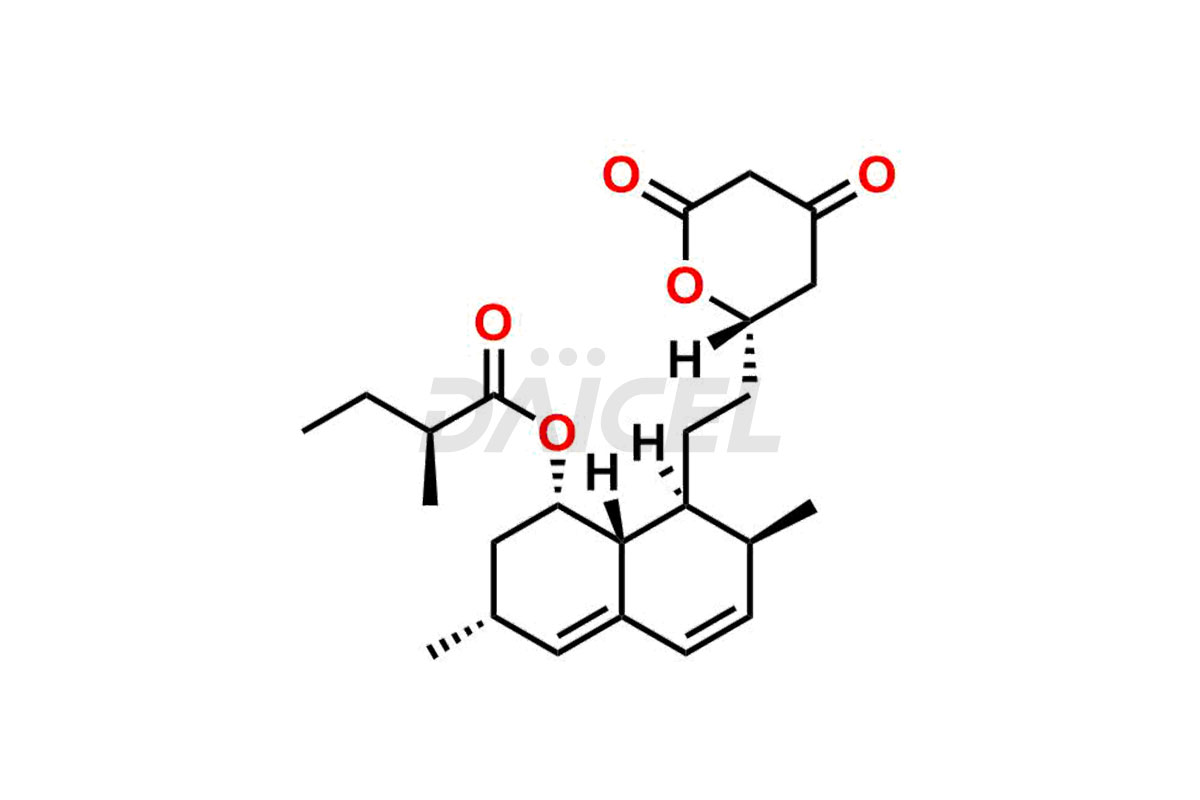
For evaluating the purity and safety of Lovastatin, an active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Lovastatin impurity standards. These impurity standards include crucial compounds such as Dehydro Lovastatin, Lovastatin hydroxy acid ammonium salt, and Lovastatin related compound #6. Additionally, Daicel Pharma provides worldwide delivery options for Lovastatin impurity standards.
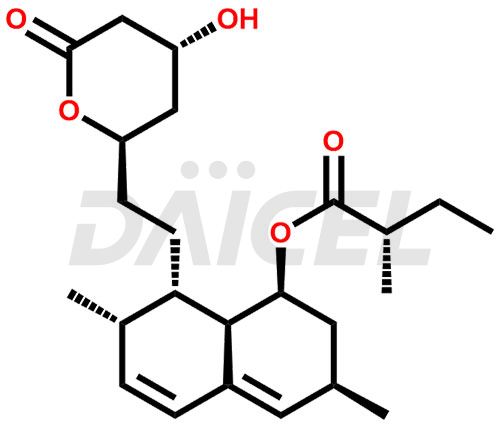
Lovastatin [CAS: 75330-75-5], derived from the fungus Aspergillus terreus, is a lactone metabolite with cholesterol-lowering properties and antineoplastic effects. As a member of the statin class of medications, it lowers cholesterol levels in the body.
Lovastatin: Use and Commercial Availability
Lovastatin, marketed under the brand names, Altoprev and Mevacor, is a statin medication used to treat and prevent various conditions related to lipid levels. It treats coronary heart disease, hypercholesterolemia. It also treats heterozygous familial hypercholesterolemia in adolescent patients. Lovastatin was isolated from a strain of Aspergillus terreus.
Lovastatin Structure and Mechanism of Action
The chemical name of Lovastatin is (1S,3R,7S,8S,8aR)-8-(2-((2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (S)-2-methylbutanoate. Its chemical formula is C24H36O5, and its molecular weight is approximately 404.5 g/mol.
Lovastatin is hydrolyzed to β-hydroxy acid, a HMG-CoA reduce inhibitor. It further converts HMG-CoA to mevalonate.
Lovastatin Impurities and Synthesis
In the pharmaceutical industry, the analysis and control of impurities in Lovastatin1, a cholesterol-lowering medication, is essential to ensure its safety and efficacy. They may include related compounds and degradation products. Analytical techniques such as high-performance liquid chromatography (HPLC) and liquid chromatography (LC) help identify and quantify these impurities. Strict control measures and specifications limit the presence of Lovastatin impurities adhering to regulatory guidelines.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for the preparation of Lovastatin impurity standards, which include Dehydro Lovastatin, Lovastatin hydroxy acid ammonium salt, and Lovastatin related compound #6. Our Lovastatin impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Lovastatin impurity standards and degradation products. Each delivery has a comprehensive characterization report, ensuring quality and transparency.
References
FAQ's
References
- Patchett, Arthur A.; Kuo, Chan Hwa, Hydrogenation Products Of Mevinolin And Dihydromevinolin, A Process For Preparing The Same And An Antihypercholesterolemic Pharmaceutical Composition Containing The Same, Merck and Co., Inc., United States, EP33537B1, May 29, 1985
- Houck, Anthony; Thomas, Scott; Ellison, Dean K., Liquid chromatographic determination of the known low-level impurities in lovastatin bulk drug: an application of high-low chromatography, Talanta, Volume: 40, Issue: 4, Pages: 491-4, 1993
Frequently Asked Questions
How are Lovastatin impurities monitored during stability testing?
Stability testing of Lovastatin involves subjecting the drug product to various conditions to evaluate its impurity profile over time. It ensures impurity levels remain within acceptable limits throughout the product's shelf life.
What is the role of validation studies in controlling Lovastatin impurities?
Validation studies are conducted to ensure that the analytical methods used for impurity analysis in Lovastatin are accurate, precise, and reliable, enabling effective control of impurities during manufacturing.
Which solvent helps in analyzing Lovastatin impurities?
Methanol is the solvent used when analyzing many impurities in Lovastatin.
What is the recommended storage temperature for Lovastatin impurities?
Lovastatin impurities should be stored, at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.