Domperidone
General Information
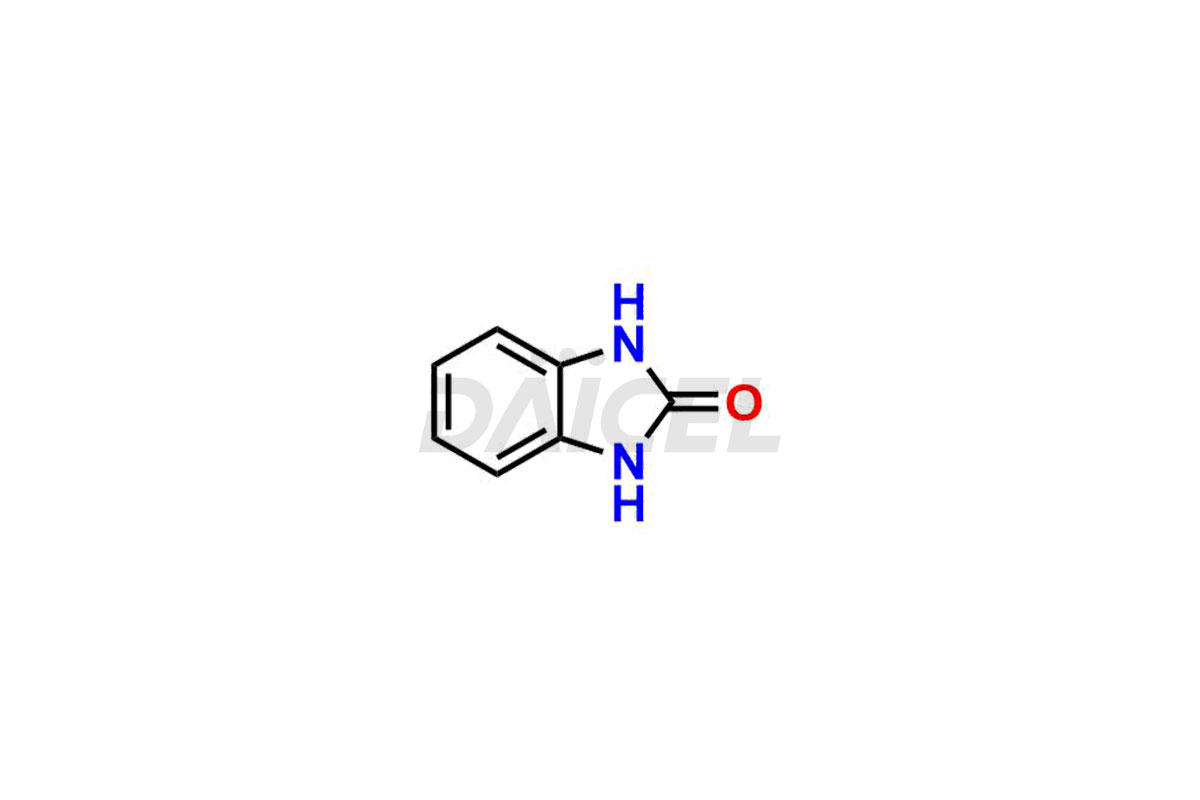
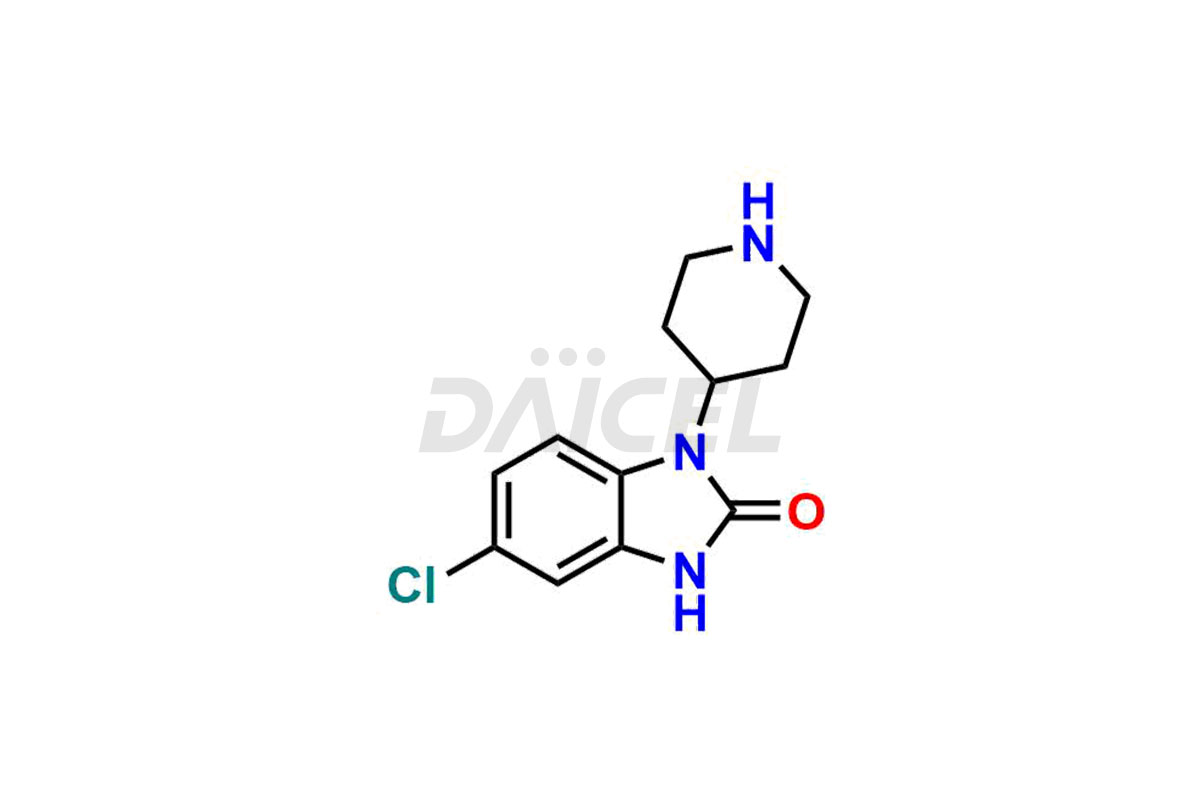
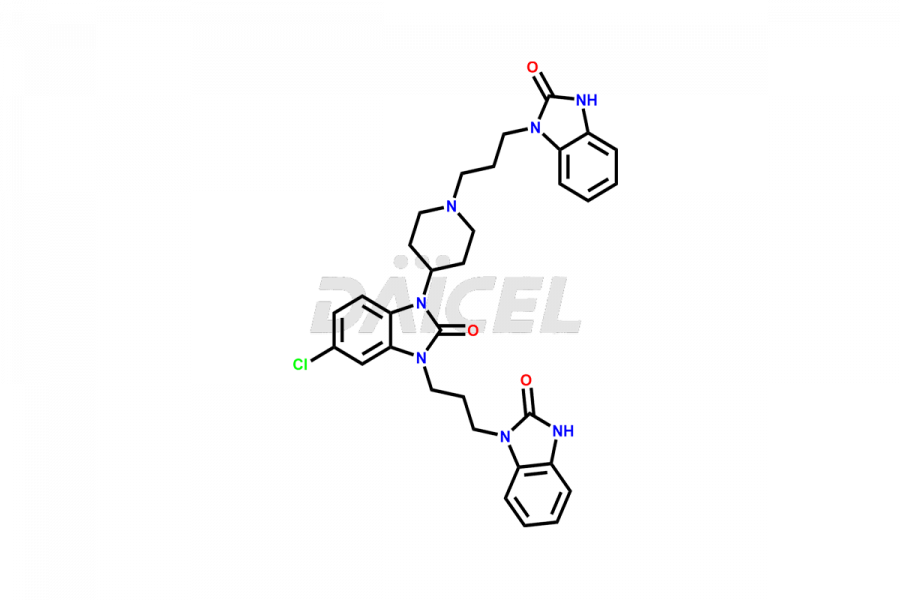
Domperidone Impurities and Domperidone
Daicel Pharma specializes in offering high-quality impurities for Domperidone, an active pharmaceutical ingredient. These impurities, including Domperidone (RC-2), Domperidone (RC-3), Domperidone (RC-4), Domperidone (RC-5), Domperidone Dimer (RC-7), and Domperidone-N-Oxide, play a vital role in assessing the purity, reliability, and safety of Domperidone. Daicel Pharma also offers a customized synthesis of Domperidone impurities to cater to client requirements, with worldwide delivery options available.
Domperidone [CAS: 57808-66-9] is a dopamine antagonist with multiple uses. It is an antiemetic and treats short-term nausea and vomiting. Additionally, it helps control the gastrointestinal effects of dopaminergic drugs used in parkinsonism management. Domperidone helps to counteract constipation in patients with Parkinson’s disease.
Domperidone: Use and Commercial Availability
Domperidone, available under Motilium, is a peripheral D2 receptor antagonist. It acts as a prokinetic and antiemetic. Domperidone increases gastrointestinal motility in the upper digestive tract and raises lower esophageal sphincter pressure. It is for diabetic and idiopathic gastroparesis treatment. While anti-nausea drugs like metoclopramide can worsen Parkinson’s disease symptoms, Domperidone can help alleviate gastrointestinal symptoms without crossing the blood-brain barrier. Additionally, it may enhance the bioavailability of levodopa, another Parkinson’s disease medication.
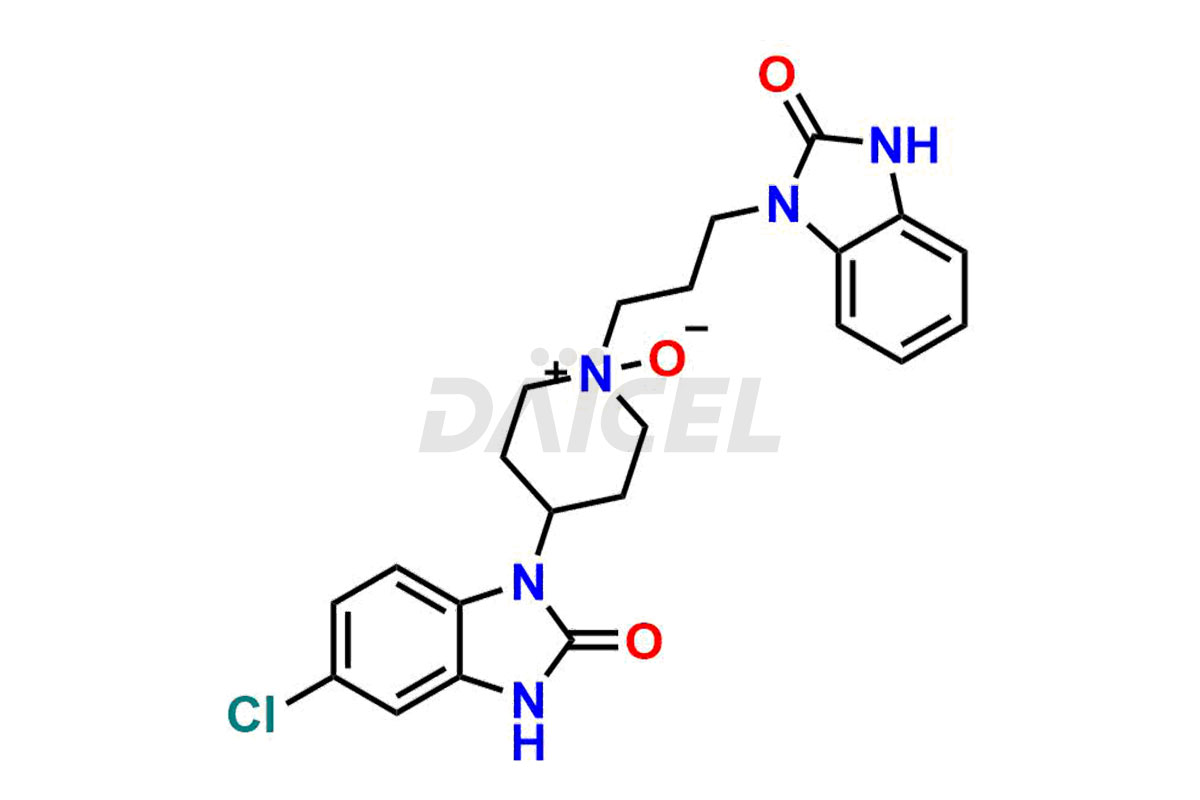
Domperidone Structure and Mechanism of Action 
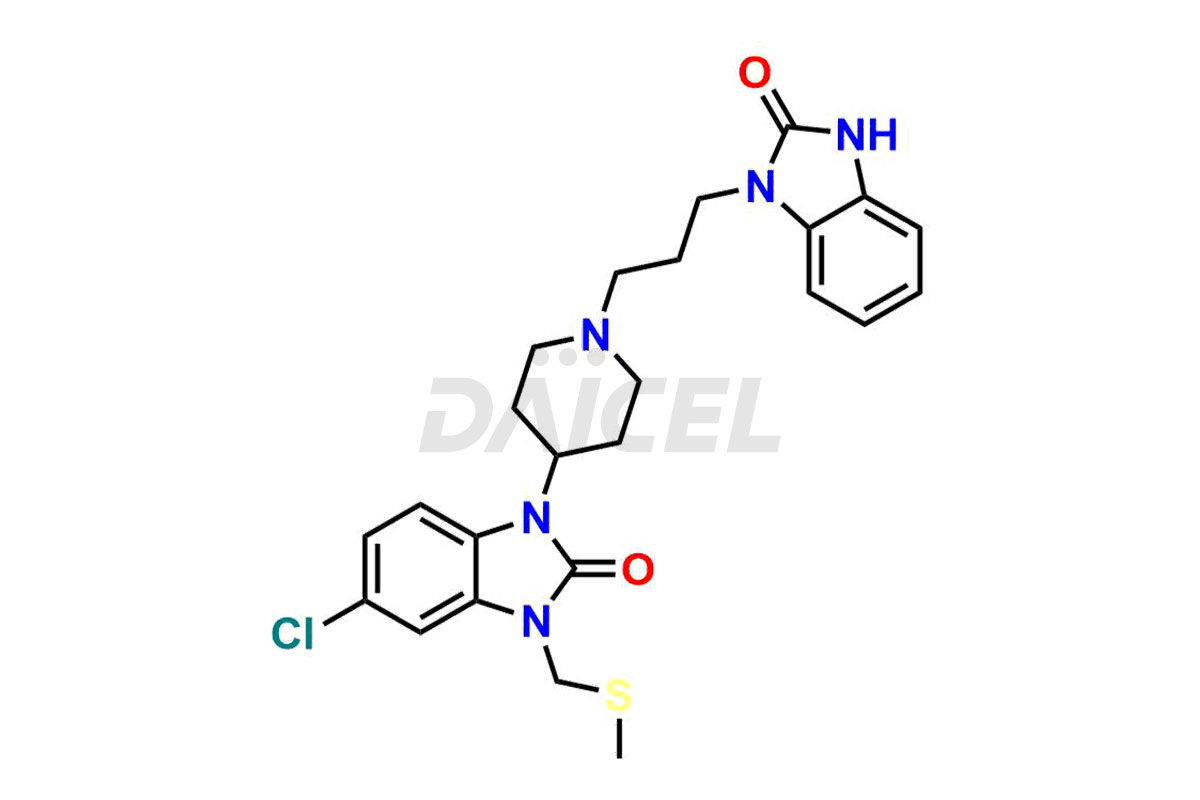
The chemical name of Domperidone is 5-Chloro-1-[1-[3-(2,3-dihydro-2-oxo-1H-benzimidazol-1-yl)propyl]-4-piperidinyl]-1,3-dihydro-2H-benzimidazol-2-one. Its chemical formula is C22H24ClN5O2, and its molecular weight is approximately 425.9 g/mol.
Domperidone blocks dopamine receptors and does not cross the blood-brain barrier.
Domperidone Impurities and Synthesis
The formation of impurities in Domperidone, a dopamine antagonist, can impact its quality, safety, and efficacy. Impurities may arise during the synthetic process1 or storage of Domperidone. It also includes degradation products, residual solvents, or related substances. Synthesis of impurities is necessary to identify and characterize them accurately. Analytical methods, such as chromatography and spectroscopy, help analyze and quantify impurities in Domperidone. Strict control measures are essential to establish acceptable limits for impurity levels, ensure the quality and purity of Domperidone for patient use, and comply with regulatory standards.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Domperidone impurity standards, including Domperidone (RC-2), Domperidone (RC-3), Domperidone (RC-4), Domperidone (RC-5), Domperidone Dimer (RC-7), and Domperidone-N-Oxide. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Domperidone impurities or degradation products, and labeled compounds, to evaluate the efficacy of generic Domperidone. Also, Domperidone-D6, a deuterium-labeled Domperidone standard, is available for bio-analytical research, including BA/BE studies. Every delivery has a complete characterization report.
References
FAQ's
References
- Vandenberk, Jan; Kennis, Ludo E. J.; Van der Aa, Marcel J. M. C.; Van Heertum, Albert A. M. Th., 1-(Benzazolylalkyl)Piperidine Derivatives, Janssen Pharmaceutica N. V., Belgium, GB1542514A, March 21, 1979 (https://portal.unifiedpatents.com/patents/patent/GB-1542514-A)
- Smit, M. J.; Sutherland, F. C. W.; Hundt, H. K. L.; Swart, K. J.; Hundt, A. F.; Els, J., Rapid and sensitive liquid chromatography-tandem mass spectrometry method for the quantitation of domperidone in human plasma, Journal of Chromatography A, Volume: 949, Issue: 1-2, Pages: 65-70, 2002
Frequently Asked Questions
How are Domperidone impurities regulated in the drug?
Regulatory authorities, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have established guidelines and standards for the control of impurities in pharmaceuticals, including Domperidone. Compliance with these regulations is mandatory for drug manufacturers.
Can Domperidone impurities vary between different manufacturers?
Impurities in Domperidone can vary between different manufacturers due to variations in the synthetic processes, starting materials, and quality control procedures used by each manufacturer. However, all manufacturers are required to comply with regulatory standards for impurity control.
How often are Domperidone impurities monitored?
Impurities in Domperidone are monitored throughout the drug's lifecycle, from development to manufacturing and post-marketing surveillance. Regular monitoring ensures compliance with regulatory standards and identifies any potential changes or new impurities.
What are the temperature conditions required to store Domperidone impurities?
Domperidone impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.