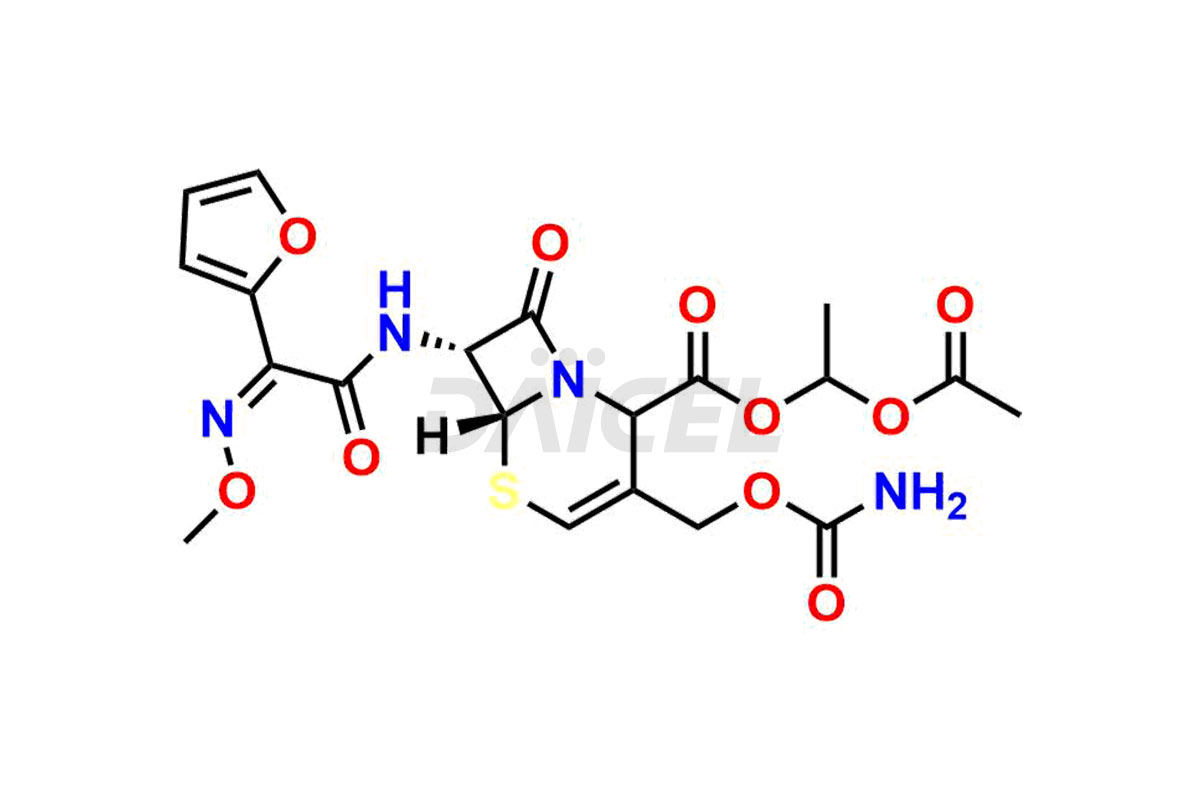
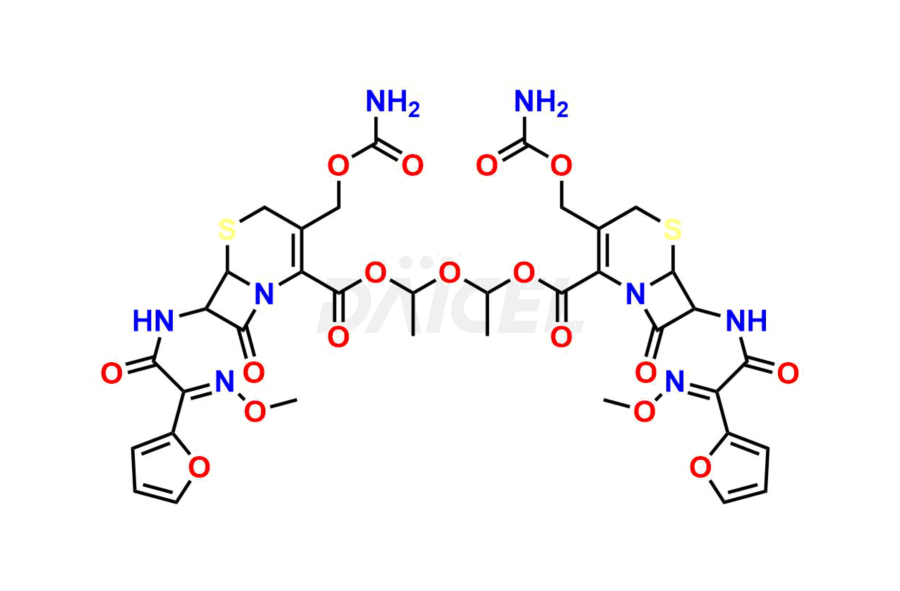
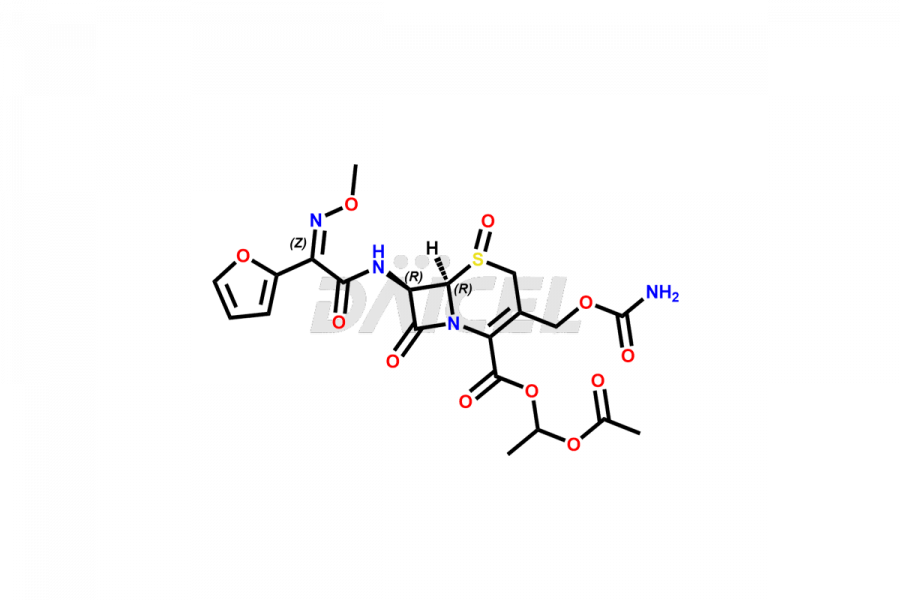
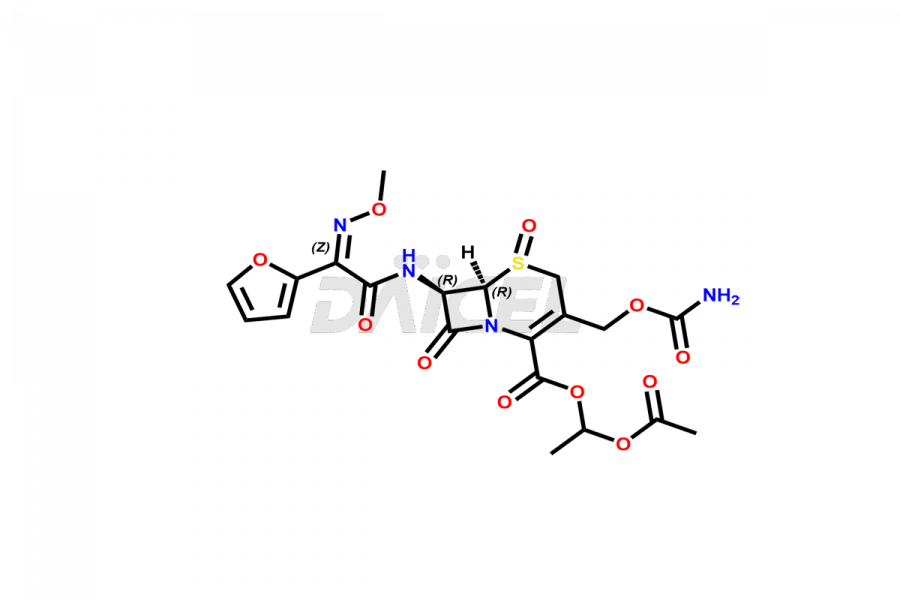
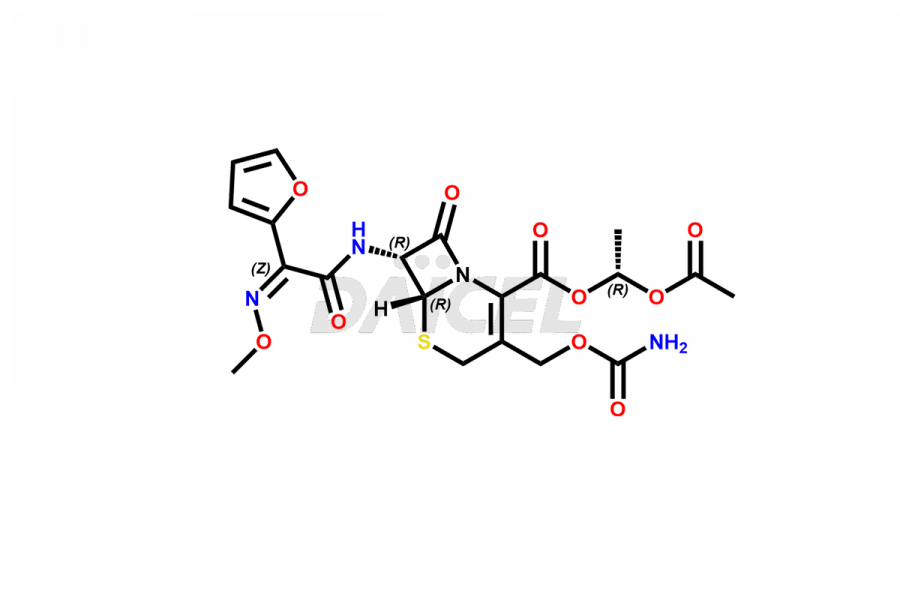
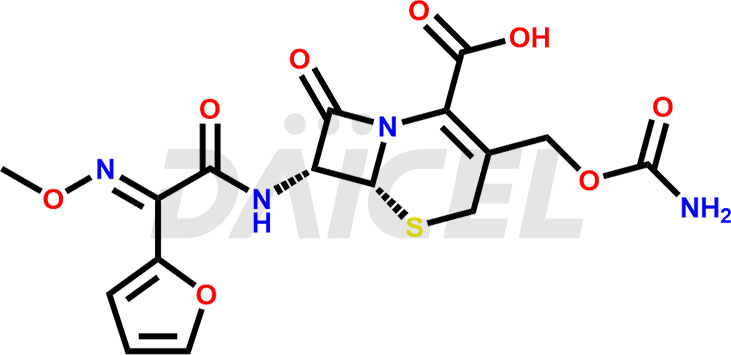
Cefuroxime
General Information
Cefuroxime Impurities and Cefuroxime
Daicel Pharma synthesizes high-quality Cefuroxime impurities, Cefuroxime axetil Delta-3 Isomers, and Cefuroxime axetil dimer, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Cefuroxime. Moreover, Daicel Pharma offers custom synthesis of Cefuroxime impurities and delivers them globally.
Cefuroxime [CAS: 55268-75-2] is an antibacterial medicine belonging to the group of second-generation cephalosporins. It is semi-synthetic and has a broad spectrum of activity against bacteria.
Cefuroxime: Use and Commercial Availability
Cefuroxime is an antibiotic that treats various bacterial infections, including ear infections, bronchitis, skin infections, sinusitis, tonsillitis, gonorrhea, and urinary tract infections. It is available under brand names such as Zinacef, Ceftin, Cefuroxime Axetil, and Cefuroxime Sodium.
Cefuroxime Structure and Mechanism of Action 
The chemical name of Cefuroxime is (6R,7R)-3-[[(Aminocarbonyl)oxy]methyl]-7-[[(2Z)-2-(2-furanyl)-2-(methoxyimino)acetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. Its chemical formula is C16H16N4O8S, and its molecular weight is approximately 424.4 g/mol.
Cefuroxime inhibits bacterial cell wall synthesis and leads to cell lysis.
Cefuroxime Impurities and Synthesis
Cefuroxime is a cephalosporin antibiotic that is prone to the formation of impurities during its manufacturing1 and storage. Impurities may form due to the degradation of the active pharmaceutical ingredient2 or through the reaction of the active ingredient with other compounds. Controlling and minimizing the formation of impurities in Cefuroxime is critical to ensure its quality and safety for use in patients.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Cefuroxime impurity standards, Cefuroxime axetil Delta-3 Isomers, and Cefuroxime axetil dimer. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity3. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Cefuroxime impurity or degradation product.
References
FAQ's
References
- Cook, Martin Christopher; Gregory, Gordon I.; Bradshaw, Janice, 7-Hydrocarbonoxy imino-acetamido-3-carbamoyloxy methylceph-3-em-4 carboxylic acids, Glaxo Laboratories, Ltd., United Kingdom, US3974153A, August 10, 1976
- Fogg, A. G.; Fayad, N. M.; Burgess, C.; McGlynn, A., Differential pulse polarographic determination of cephalosporins and their degradation products, Analytica Chimica Acta, Volume: 108, Pages: 205-11, 1979,
- Coomber, Patricia A.; Jefferies, J. P.; Woodford, J. D., High-performance liquid chromatographic determination of cefuroxime, Analyst (Cambridge, United Kingdom), Volume: 107, Issue: 1281, Pages: 1451-6, 1982
Frequently Asked Questions
How can the presence of Cefuroxime impurities affect the efficacy of the drug?
Cefuroxime impurities can affect the drug's efficacy by interfering with its pharmacological activity or causing adverse effects on health.
How are Cefuroxime impurities controlled during the manufacturing process?
Cefuroxime impurities are controlled during the manufacturing process by implementing measures such as process optimization, impurity profiling, and quality control testing.
Which solvent is used for the analysis of Cefuroxime impurities?
Acetonitrile is used as a solvent in analyzing Cefuroxime impurities.
What are the temperature conditions required to store Cefuroxime impurities?
Cefuroxime impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.