Atenolol
General Information
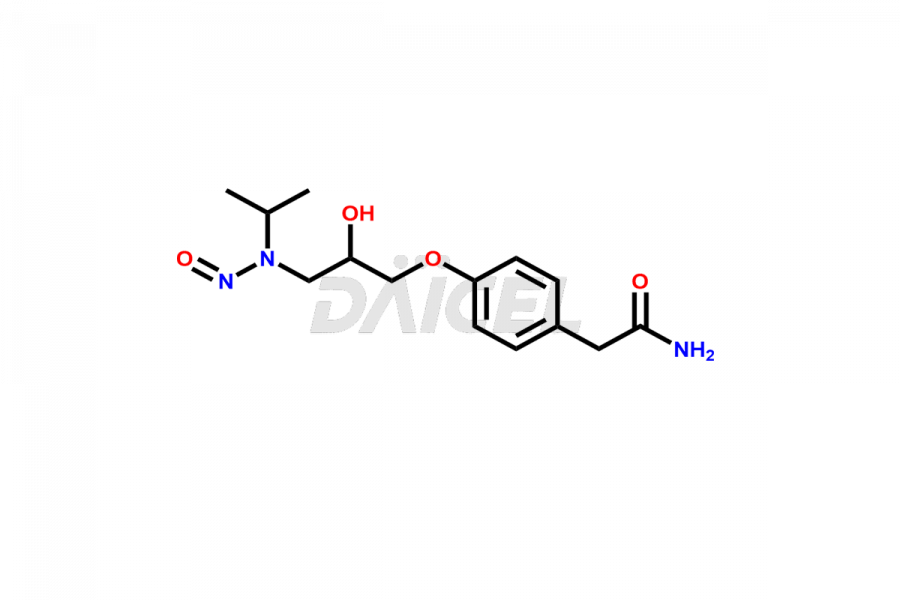
Atenolol Impurities and Atenolol
Daicel Pharma offers superior-quality Atenolol impurities, such as N-Nitroso Atenolol Impurity. It is vital to evaluate Atenolol quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of atenolol impurities and ensures their worldwide delivery.
Atenolol [CAS: 29122-68-7] is an ethanolamine derivative and is an antihypertensive medicine. It is a synthetic, second-generation, beta1-selective adrenoreceptor blocking agent. It helps lower blood pressure and myocardial contractility in patients.
Atenolol: Use and Commercial Availability
As an adrenergic beta-blocker, Atenolol reduces the risk of cardiovascular disease in patients. It is a US FDA-approved drug for treating angina pectoris, hypertension, and myocardial infarction. It is used alone as a monotherapy or combined with other antihypertensive drugs. Further, it can treat arrhythmias and prevent migraines. It can also manage supraventricular tachycardia, atrial fibrillation, and alcohol withdrawal in patients. Various generic manufacturers prepare Atenolol. It is available orally under brands such as Tenormin, Prenormine, Atenil, Betacard, etc.
Atenolol Structure and Mechanism of Action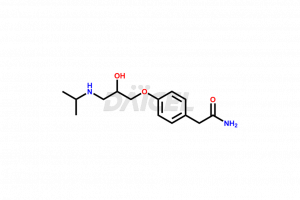
The chemical name of Atenolol is 4-[2-Hydroxy-3-[(1-methylethyl)amino]propoxy]benzeneacetamide. The chemical formula for Atenolol is C14H22N2O3, and its molecular weight is approximately 266.34 g/mol.
Atenolol binds selectively to the beta-1 adrenergic receptors in the heart and vascular smooth muscles. It inhibits sympathetic stimulation, causing a decrease in heart rate and blood pressure.
Atenolol Impurities and Synthesis
While synthesizing Atenolol1, impurities may form that will affect the safety and efficacy of the drug. These impurities form during Atenolol synthesis, purification, storage, or degradation. Controlling and monitoring Atenolol impurities improves the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Atenolol impurities, which includes N-Nitroso Atenolol Impurity. A CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data2 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity3. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Atenolol impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable deuterium-labeled standard of N-Nitroso Atenolol-d5. A complete characterization report is available on delivery.
References
FAQ's
References
- Barrett, Arthur M.; Hull, Roy; Le Count, David J.; Squire, Christopher J.; Carter, John, Alkanolamine derivatives, GB1285038A, Aug 9, 1972, Imperial Chemical Industries Ltd., United Kingdom (https://www.lens.org/lens/search/patent/list?q=GB1285038)
- Scales, Bryan; Copsey, Paul B, Gas chromatographic determination of atenolol in biological samples, Journal of Pharmacy and Pharmacology, Volume: 27, Issue: 6, Pages: 430-3, 1975 DOI: (1111/j.2042-7158.1975.tb09473.x)
- Weddle, Orville H.; Amick, Edwin N.; Mason, William D., Rapid determination of atenolol in human plasma and urine by high-pressure liquid chromatography, Journal of Pharmaceutical Sciences, Volume: 67, Issue: 7, Pages: 1033-5, 1978 DOI: (10.1002/jps.2600670748)
Frequently Asked Questions
2.How do we analyze Atenolol impurities in the drug substance?
HPLC method analyzes Atenolol impurities in the drug substance.
3.How do we control Nitrosamine Impurities in Atenolol?
During process development, manufacturers can avoid reactions that produce nitrosamines, use amide solvents, and monitor any cross-contamination of materials. Further, the control strategy should have specification limits to detect nitrosamine levels in Atenolol.
4.What is the source of Atenolol impurities?
The source of Atenolol impurities includes unreacted starting materials, by-products, intermediates, solvents, and reagents.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.