Vericiguat
General Information
Vericiguat Impurities and Vericiguat
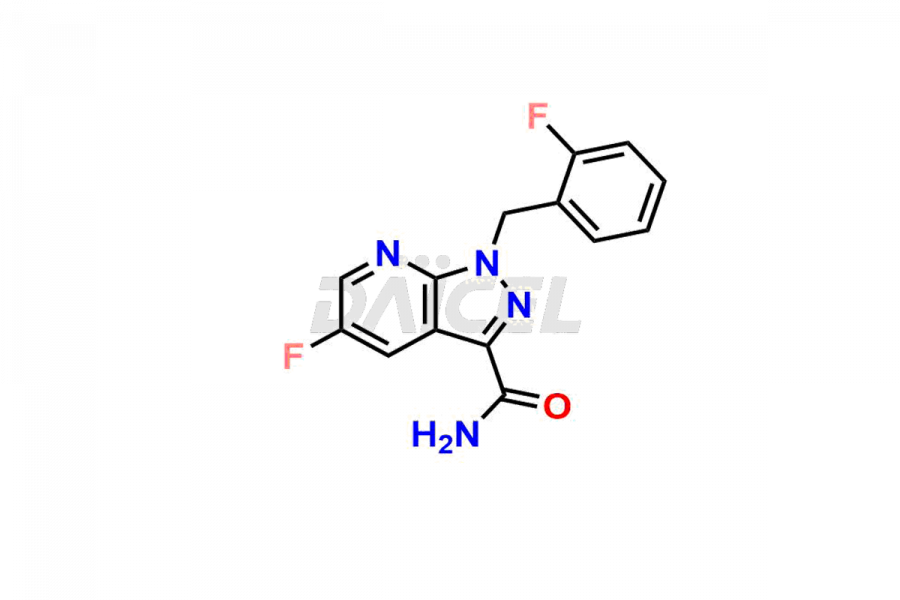
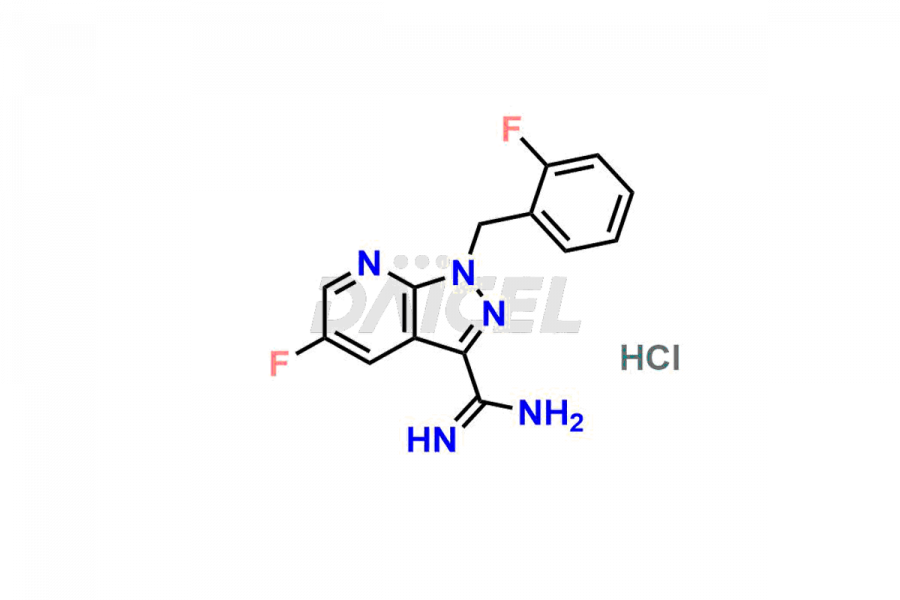
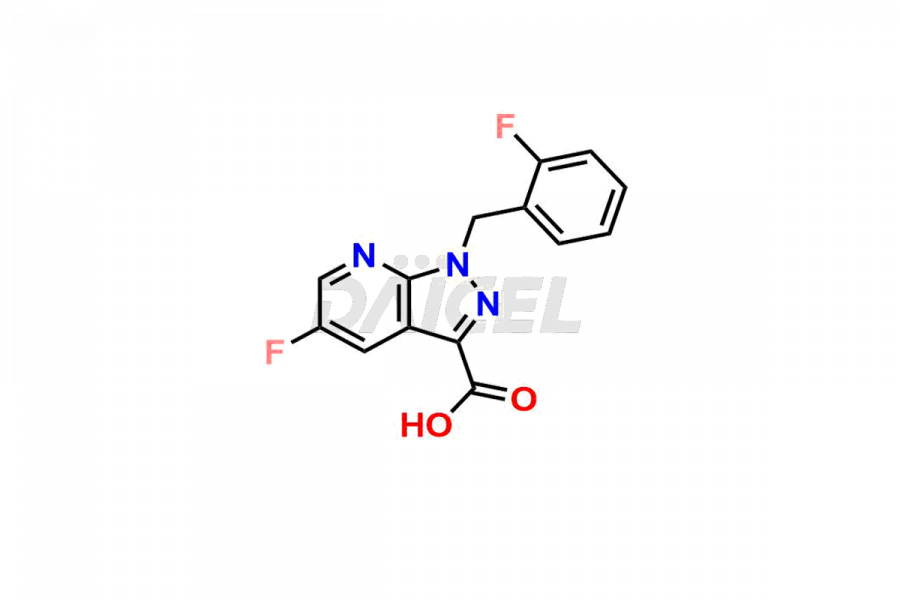
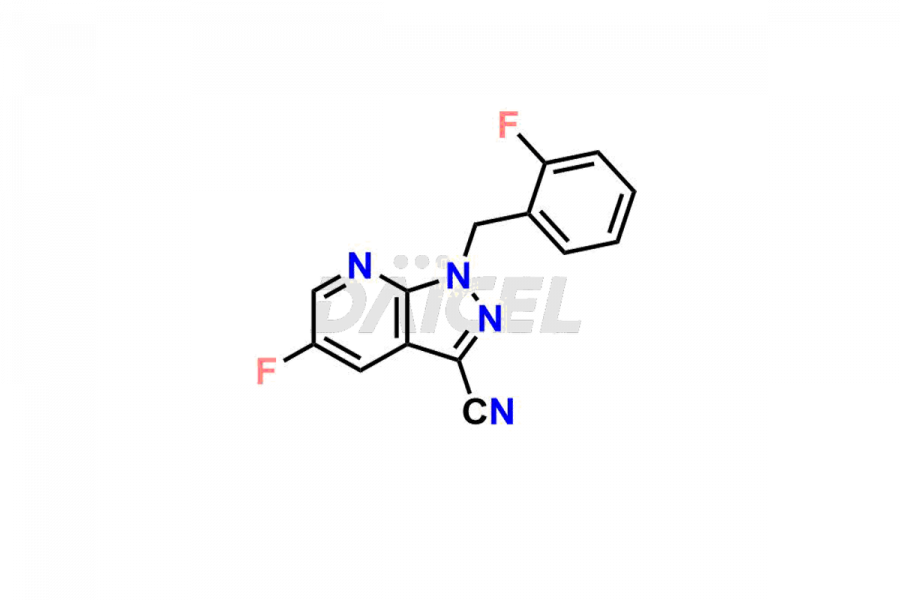
Daicel Pharma is a reliable partner for synthesizing high-quality Vericiguat impurities, specifically 5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamide, Vericiguat Carboxidamide Hydrochloride Impurity, Vericiguat Carboxylic Acid Impurity, and Vericiguat Nitrile Impurity. These impurities help assess the quality, stability, and safety of the active pharmaceutical ingredient, Vericiguat. Daicel Pharma also offers a custom synthesis of Vericiguat impurities, which can be shipped worldwide to meet customers’ unique requirements.
Vericiguat [CAS: 1350653-20-1] is a medication that manages and treats heart failure with reduced ejection fraction (HFrEF). It is a novel oral soluble guanylate cyclase stimulator for treating chronic heart failure.
Vericiguat: Use and Commercial Availability
Vericiguat reduces the risk of cardiovascular death and heart failure-related hospitalization in adults with symptomatic, chronic heart failure. Vericiguat prevents rehospitalization for heart failure patients. The tradename for Vericiguat is Verquvo.
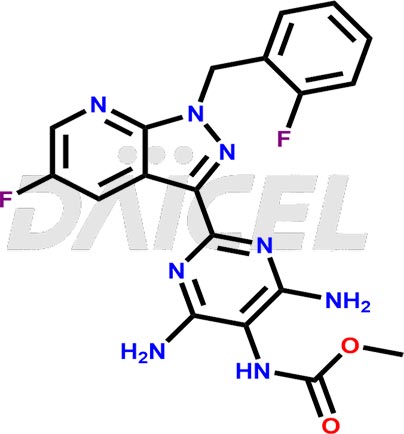
Vericiguat Structure and Mechanism of Action 
The chemical name of Vericiguat is Methyl [4,6-diamino-2-[5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]carbamate. Its chemical formula is C19H16F2N8O2, and its molecular weight is approximately 426.4 g/mol.
Vericiguat stimulates soluble guanylate cyclase (sGC), an enzyme in the Nitric Oxide (NO) signaling pathway. The enzyme increases the level of intracellular cGMP resulting in smooth muscle relaxation and vasodilation.
Vericiguat Impurities and Synthesis
Vericiguat impurities can arise from various sources, such as starting materials, synthetic reactions, and storage conditions. The most common impurities in Vericiguat are related to its synthetic process1 and may include byproducts, degradation products, and solvents. These impurities can impact drug stability, pharmacokinetics, and toxicity. Therefore, it is crucial to control Vericiguat impurities by implementing appropriate manufacturing processes, analytical methods, and quality standards.
Daicel Pharma provides a Certificate of Analysis (CoA) for Vericiguat impurity standards, which includes 5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamide, Vericiguat Carboxidamide Hydrochloride Impurity, Vericiguat Carboxylic Acid Impurity, and Vericiguat Nitrile Impurity. The CoA is from an analytical facility that adheres to current Good Manufacturing Practices (cGMP) and includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization data such as 13C-DEPT and CHN on request. Daicel Pharma can also generate unknown Vericiguat impurities or degradation products and provide labeled compounds to assess the effectiveness of Vericiguat. Further, Daicel Pharma offers Vericiguat-D3 HCl, a deuterium-labeled Vericiguat compound used in bio-analytical research, such as BA/BE studies. A complete characterization report is a part of each delivery.
References
FAQ's
References
- Hirth-Dietrich, Claudia; Sandner, Peter; Stasch, Johannes-Peter; Knorr, Andreas; Von Degenfeld, Georges; Hahn, Michael; Follmann, Markus, Substituted 5-fluoro-1H-pyrazolopyridines and their use, Bayer Pharma AG, US8420656B2, April 16, 2013
- Varadkar, Anuja; Patankar, Shivani; Sonawane, Bhushan; Munipalli, Vijay Kumar; Warde, S. U.; Singh, Raman Mohan; Dhume, Naomita, Development and validation of HPTLC method for estimation of vericiguat in tablet dosage form, World Journal of Pharmacy and Pharmaceutical Sciences, Volume: 12, Issue: 1, Pages: 1368-1378, 2023
Frequently Asked Questions
What are the main Vericiguat impurities?
The main impurities in Vericiguat are related substances, which include impurities resulting from the synthetic process, degradation products, and residual solvents.
What analytical methods help detect Vericiguat impurities?
Various analytical methods help detect impurities in Vericiguat, including chromatography and mass spectrometry. High-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS) are the methods for separating and quantifying impurities.
How is the stability of Vericiguat affected by impurities?
The stability of Vericiguat may be affected by impurities, particularly degradation products. Impurities can cause changes in the physical, chemical, or biological properties of Vericiguat, leading to reduced potency and shelf life.
What are the temperature conditions required to store Vericiguat impurities?
Vericiguat impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.