LOAD MORE
You're viewed 9 of 21 products
Daicel Pharma is a reliable source for synthesizing high-quality Tetrabenazine impurity standards, specifically (+) α-Dihydro tetrabenazine, (+) β -Dihydro tetrabenazine, Tetrabenazine Related Impurity 3, and more. These impurities are essential in accurately analyzing Tetrabenazine quality, stability, and biological safety. Additionally, Daicel Pharma offers a customized synthesis of Tetrabenazine impurities for global delivery to meet the specific needs of our customers.
Tetrabenazine [CAS: 58-46-8] treats hyperkinetic movement disorders such as chorea in Huntington’s disease, Tourette syndrome, tardive dyskinesia, hemiballismus, and senile chorea.
Tetrabenazine is an inhibitor of the human vesicular monoamine transporter type 2. It manages hyperkinetic movement disorders and tardive dyskinesia characterized by involuntary movements.
Tetrabenazine is available under Xenazine, which contains the active ingredient, Tetrabenazine.
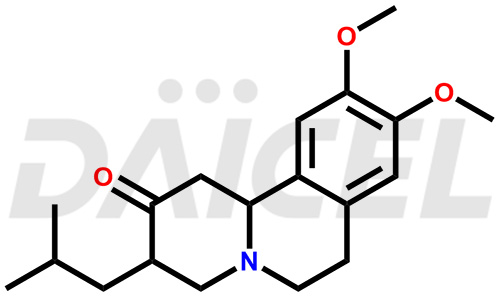
The chemical name of Tetrabenazine is rel-(3R,11bR)-1,3,4,6,7,11b-Hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-benzo[a]quinolizin-2-one. Its chemical formula is C19H27NO3, and its molecular weight is approximately 317.4 g/mol.
The precise mechanism of action of Tetrabenazine is unknown.
During the Tetrabenazine preparation1, impurity formation is possible, compromising its effectiveness. They can arise from various sources, including the raw materials, intermediates, and chemicals utilized to synthesize Tetrabenazine. Close management and monitoring of impurities ensure the drug’s efficacy and safety.
Daicel offers a wholesome and integrated Certificate of Analysis (CoA) for Tetrabenazine impurity standards, encompassing (+) α-Dihydro tetrabenazine, (+) β -Dihydro tetrabenazine, Tetrabenazine Related Impurity 3, and more. The CoA provides detailed characterization data, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Additionally, we give a detailed 13C-DEPT on delivery. With advanced technology and expertise, Daicel can synthesize any unknown Tetrabenazine impurity or degradation products. We also supply labeled compounds, facilitating the quantification of generic Tetrabenazine efficacy. For bioanalytical research and BA/BE studies, we also offer deuterium-labeled Tetrabenazine standards, 9-Desmethyl Tetrabenazine-D3, 9-desmethyl α-dihydro tetrabenazine D3, Deutetrabenazine DTBRC-4, Dihydrodeutetrabenazine-D6, α-Dihydro Deutetrabenazine, and β-Dihydro Deutetrabenazine.
Analytical testing plays a crucial role in identifying impurities in Tetrabenazine by delivering precise and dependable data regarding their existence and concentration.
The impurities in Tetrabenazine are controlled during storage by ensuring appropriate temperature, humidity, and light conditions and using proper packaging and storage containers.
Tetrabenazine impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
The presence of impurities in Tetrabenazine can impact its therapeutic efficacy by diminishing the drug's potency or modifying its mode of action.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.