Tapentadol
General Information
Tapentadol Impurities and Tapentadol
Daicel Pharma offers a diverse range of Tapentadol impurities, such as 3-(3-methoxyphenyl)-N, N,2-trimethylpentan-1-amine, Hydroxy Tapentadol N-Oxide, Tapentadol EP Impurity E, and Tapentadol Impurity A. They help assess the quality, stability, and biological safety of the active pharmaceutical ingredient Tapentadol. Additionally, Daicel Pharma can synthesize Tapentadol impurities according to precise customer specifications, guaranteeing reliable worldwide delivery.
Tapentadol [CAS: 175591-23-8] is an analgesic that alleviates moderate to severe pain that occurs suddenly. The long-acting formulation of this drug provides consistent and ongoing pain relief for individuals experiencing moderate to severe chronic or neuropathic pain linked to diabetic peripheral neuropathy.
Tapentadol: Use and Commercial Availability
Tapentadol treats severe to moderate acute pain. Tapentadol is also a valuable analgesic for treating acute, chronic, and neuropathic pain.
Tapentadol is available under Nucynta and Nucynta ER, which effectively contain the active ingredient Tapentadol.
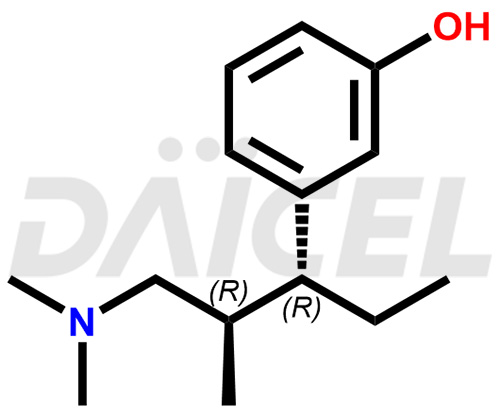
Tapentadol Structure and Mechanism of Action 
The chemical name of Tapentadol is 3-[(1R,2R)-3-(Dimethylamino)-1-ethyl-2-methylpropyl]phenol. Its chemical formula is C14H23NO, and its molecular weight is approximately 221.34 g/mol
Tapentadol has mu-opioid agonist activity and inhibits norepinephrine uptake for analgesic efficacy.
Tapentadol Impurities and Synthesis
During the synthesis1 of Tapentadol, various impurities like related substances, degradation products, and residual solvents form. It is vital to carefully monitor and control these impurities to ensure the drug’s safety, efficacy, and overall quality.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Tapentadol impurity standards, which includes 3-(3-methoxyphenyl)-N, N,2-trimethylpentan-1-amine, Hydroxy Tapentadol N-Oxide, Tapentadol EP Impurity E, and Tapentadol Impurity A. They are manufactured in compliance with current Good Manufacturing Practices (cGMP). The CoA provides detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2, offering a thorough understanding of the impurity profile. If required, Daicel can also provide 13C-DEPT data for further characterization.
Moreover, Daicel Pharma possesses the technical expertise to synthesize any unknown impurities or degradation products of Tapentadol.
References
FAQ's
References
Frequently Asked Questions
How are Tapentadol impurities detected and quantified?
High-Performance Liquid Chromatography (HPLC) method can detect impurities effectively in Tapentadol.
Can Tapentadol impurities affect patient safety?
Tapentadol impurities can affect patient safety as the type and level of these impurities can lead to adverse effects or diminish the effectiveness of the medication.
Which solvents help in the analysis of Tapentadol impurities?
Methanol is used to achieve optimal solubility and separation of Tapentadol impurities.
What are the temperature conditions required to store Tapentadol impurities?
Tapentadol impurities should be stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.