Ropinirole
General Information
Ropinirole Impurities and Ropinirole
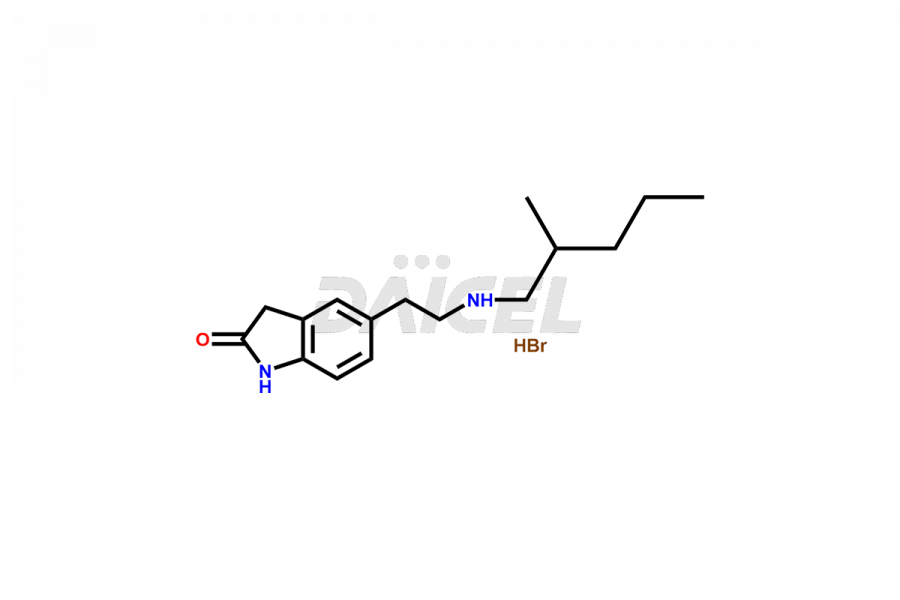
Daicel Pharma offers superior-quality Ropinirole impurities, such as Ropinirole Isohexyl Analog Hydrobromide and Ropinirole Isohexyl Analog Hydrochloride. It is vital for evaluating the quality, stability, and biological safety of Ropinirole. In addition, Daicel Pharma specializes in the custom synthesis of Ropinirole impurities and ensures their worldwide delivery.
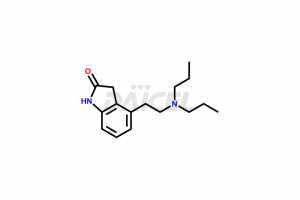
Ropinirole [CAS: 91374-21-9] is an anti-Parkinsonism drug. It is a dopamine agonist with activity at D2 and D3 receptor subtypes. Ropinirole also treats moderate to severe primary RLS (Restless Legs Syndrome).
Ropinirole: Use and Commercial Availability
Ropinirole treats Parkinson’s disease (PD) and Restless Legs Syndrome (RLS). It aids in treating early and advanced stages of Parkinson’s disease. Further, it helps patients with symptoms of sleep disturbances associated with Parkinson’s disease. Ropinirole alone or in combination with drugs like L-dopa can treat PD. Ropinirole is administered orally to patients. Requip and Requip XL are the brand names of Ropinirole.
Ropinirole Structure and Mechanism of Action
The chemical name of Ropinirole is 4-[2-(Dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one. The chemical formula for Ropinirole is C16H24N2O, and its molecular weight is approximately 260.38 g/mol.
Ropinirole stimulates the postsynaptic dopamine D2 receptors in the brain, the central and peripheral nervous system. Further, it activates potassium channels while inhibiting calcium channels and adenylyl cyclase.
Ropinirole Impurities and Synthesis
During the synthesis of Ropinirole 1, impurities may form that may affect the safety and efficacy of the drug. These impurities form during the synthesis, storage, or degradation of Ropinirole. Ropinirole impurities need control and monitoring to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Ropinirole impurities, which includes Ropinirole Isohexyl Analog Hydrobromide and Ropinirole Isohexyl Analog Hydrochloride. An issued CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data2,3 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Ropinirole impurity or degradation product. In addition, Daicel Pharma offers highly purified Stable isotope-labeled standards of Ropinirole for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- Gallagher, Gregory, Jr., 4-Aminoalkyl-2(3H)-indolones, US4452808A, Jun 5, 1984, SmithKline Beckman Corp., United States (https://patents.google.com/patent/US4452808A/en)
- Coufal, Pavel; Stulik, Karel; Claessens, Henk A.; Hardy, Martin J.; Webb, Michael, Determination of the dissociation constants of ropinirole and some impurities and their quantification using capillary zone electrophoresis, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 720, Issue: 1 + 2, Pages: 197-204, 1998 DOI: (10.1016/s0378-4347(98)00427-7)
- Coufal, P.; Stuli, K.; Claessens, H. A.; Hardy, M. J.; Webb, M., Separation and quantification of ropinirole and some impurities using capillary liquid chromatography, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 732, Issue: 2, Pages: 437-444,1999 DOI: (10.1016/s0378-4347(99)00314-x)
Frequently Asked Questions
Which is the potential degradant Ropinirole Impurity?
N-Methyl Hydroxy Ropinirole is a potential degradant Ropinirole Impurity.
Which is the analytical method to detect Ropinirole impurities?
Reverse-phase high-performance liquid chromatography (RP-HPLC) helps to detect Ropinirole impurities.
Why is it essential to remove Ropinirole impurities during drug manufacturing?
The presence of Ropinirole impurities in drugs can prove toxic to humans, so it is necessary to eliminate Ropinirole impurities during drug manufacturing.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.