LOAD MORE
You're viewed 9 of 17 products
Daicel Pharma synthesizes Ribociclib impurity standards such as Ribociclib Piperizine Impurity, N-Trityl Ribociclib, 5-bromo-4-chloro-N-cyclopentylpyrimidin-2-amine, Amino Trityl Impurity, Triphenyl Iso-Propyl Ether Impurity, Amine Impurity, and Chloroamide Impurity, etc. The presence of these impurities is essential for the safety, stability, quality, and analysis of Ribociclib. Furthermore, Daicel Pharma offers custom synthesis for Ribociclib impurities, as per the client’s need, with an international delivery facility.
Ribociclib [CAS: 1211441-98-3] is a medication used to treat advanced breast cancer.
Ribociclib is a selective cyclin-dependent kinase inhibitor, a type of cancer medication that blocks two proteins called cyclin-dependent kinase 4 and 6 (CDK4/6). It treats women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2) negative advanced or metastatic breast cancer. This drug is available in the market under the tradename of Kisqali.
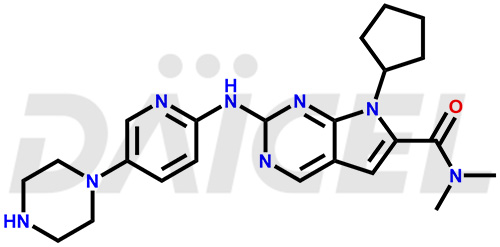
The chemical name of Ribociclib is 7-Cyclopentyl-N, N-dimethyl-2-[[5-(1-piperazinyl)-2-pyridinyl]amino]-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide. Its chemical formula is C23H30N8O, and its molecular weight is approximately 434.5 g/mol.
Ribociclib blocks CDK4/6 with great accuracy so that cancer cells do not proliferate uncontrolled.
Ribociclib impurities are chemical compounds that occur unintentionally during the production1 or storage of Ribociclib, a drug used to treat certain kinds of breast cancer. These impurities might come from raw materials, reagents, intermediates, or degradation products. Ribociclib synthesis entails several synthetic stages and purification techniques to achieve the purest form of the drug.
Daicel Pharma offers a Certificate of Analysis (CoA) for Ribociclib impurity standards such as Ribociclib Piperizine Impurity, N-Trityl Ribociclib, 5-bromo-4-chloro-N-cyclopentylpyrimidin-2-amine, Amino Trityl Impurity, Triphenyl Iso-Propyl Ether Impurity, Amine Impurity, Chloroamide Impurity, etc. Daicel Pharma’s cGMP-certified analytical laboratory provides comprehensive CoA with detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. More characterization details, such as those for 13C-DEPT, can be provided on request. Daicel Pharma specializes in synthesizing impurities and degradation products. At Daicel Pharma, we can provide Ribociclib-D8, a pure deuterium-labeled Ribociclib standard, crucial for bioanalytical research and Bioavailability/Bioequivalence (BA/BE) studies.
Ribociclib impurities are detected and analyzed using analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS).
There are specific Ribociclib impurities of particular concern due to their potential impact on patient safety.
Measures such as careful selection of starting materials, optimization of reaction conditions, and implementation of purification techniques help control and minimize the presence of Ribociclib impurities during its synthetic process.
Ribociclib impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.