LOAD MORE
You're viewed 9 of 25 products
Daicel Pharma specializes in providing Prednisolone impurity standards such as 1,2-Dihydro Loteprednol, Difluoroprednisolone-3(10H) one, Prednisolone 1,2-Propane diol protected Impurity, Prednisolone 17B hydroxy acid, Prednisolone di carbonate 1NH, Prednisolone-21-ethylcarbonate, Prednisolone 20 ethyl ester 1NH, Prednisolone Ph. Eur. Impurity E, Prednisolone Z-enol aldehyde Impurity, and more. Their presence can impact the efficacy, quality, and safety of Prednisolone. Daicel Pharma custom prepares Prednisolone impurities and delivers them globally.
Prednisolone [CAS: 50-24-8] is a glucocorticoid derived from prednisone. It functions as an adrenergic agent, anti-inflammatory medication, antineoplastic treatment, and drug metabolite. Prednisolone treats many conditions, such as allergic disorders, skin conditions, autoimmune diseases, respiratory diseases, and types of cancers.
Prednisolone treats various medical conditions, including endocrine, rheumatic, and hematologic disorders, collagen and dermatologic diseases, ophthalmic and respiratory ailments, gastrointestinal disorders, allergic reactions, edematous states, and additional conditions such as tuberculous meningitis. This drug is available under various tradenames like Cortalone, Fernisolone-P, Hydeltra-TBA, Meti-Derm, Meticortelone, Omnipred, Pred Forte, Pred Mild, Prelone, Sterane, and many more.
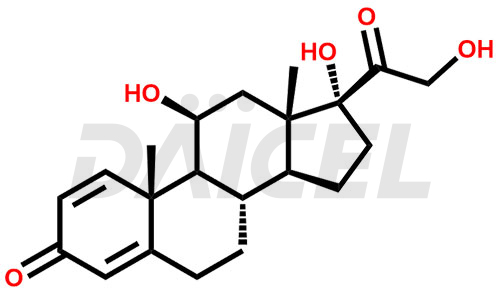
The chemical name of Prednisolone is (11β)-11,17,21-Trihydroxypregna-1,4-diene-3,20-dione. Its chemical formula is C21H28O5, and its molecular weight is approximately 360.4 g/mol.
The mechanism of action of Prednisolone is poorly understood.
Prednisolone impurities are unwanted substances in drug formulations, either as byproducts of the synthetic process1 or as impurities introduced during manufacturing or storage. These impurities can affect the quality, safety, and efficacy of Prednisolone.
Daicel Pharma provides a Certificate of Analysis (CoA) for Prednisolone impurity standards such as 1,2-Dihydro Loteprednol, Difluoroprednisolone-3(10H) one, Prednisolone 1,2-Propane diol protected Impurity, Prednisolone 17B hydroxy acid, Prednisolone di carbonate 1NH, Prednisolone-21-ethylcarbonate, Prednisolone 20 ethyl ester 1NH, Prednisolone Ph. Eur. Impurity E, Prednisolone Z-enol aldehyde Impurity, and more. Daicel Pharma offers a cGMP-certified analytical laboratory that provides the CoA, which includes thorough characterization data including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. On request, we give more characterization details, such as those for 13C-DEPT. At Daicel Pharma, we synthesize Prednisolone impurities.
Prednisolone impurities can affect its pharmacological activity by altering the drug's potency, efficacy, bioavailability, and potential for adverse effects.
Yes, impurities in Prednisolone can potentially impact its stability or shelf life.
Impurities in Prednisolone can compromise its quality and safety by potentially altering its pharmacological activity, causing adverse effects, and reducing therapeutic efficacy.
Prednisolone impurities are stored preferably at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.