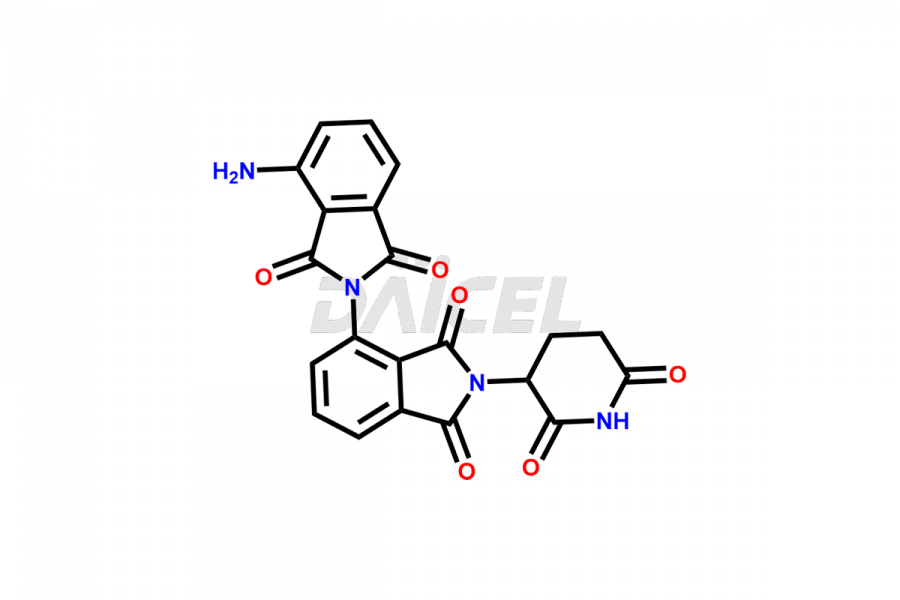
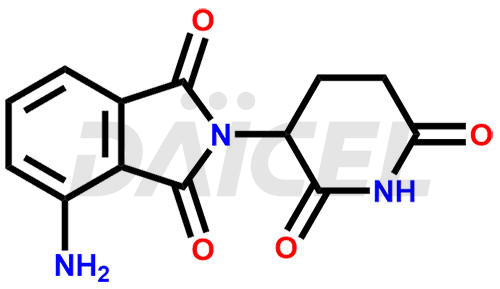
Pomalidomide
General Information
Pomalidomide Impurities and Pomalidomide
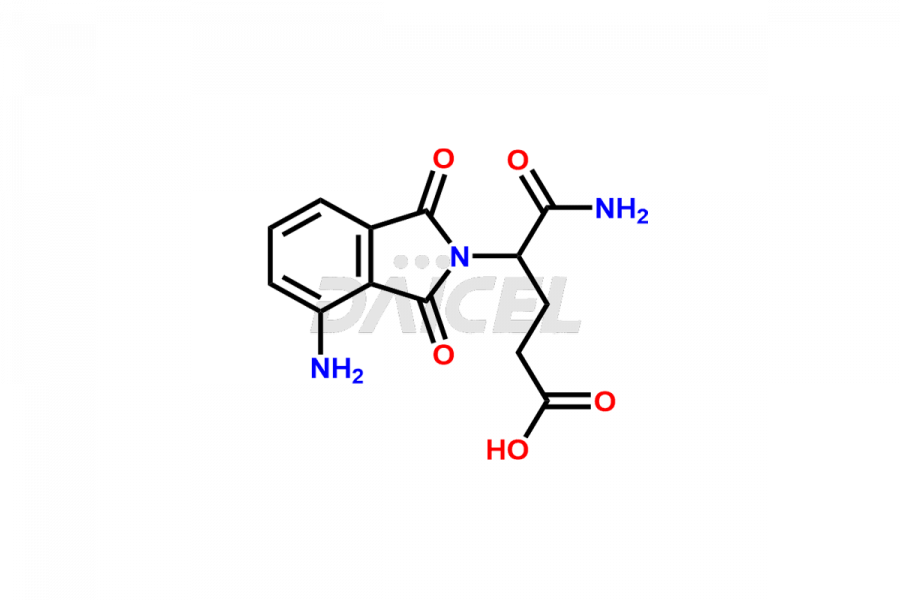
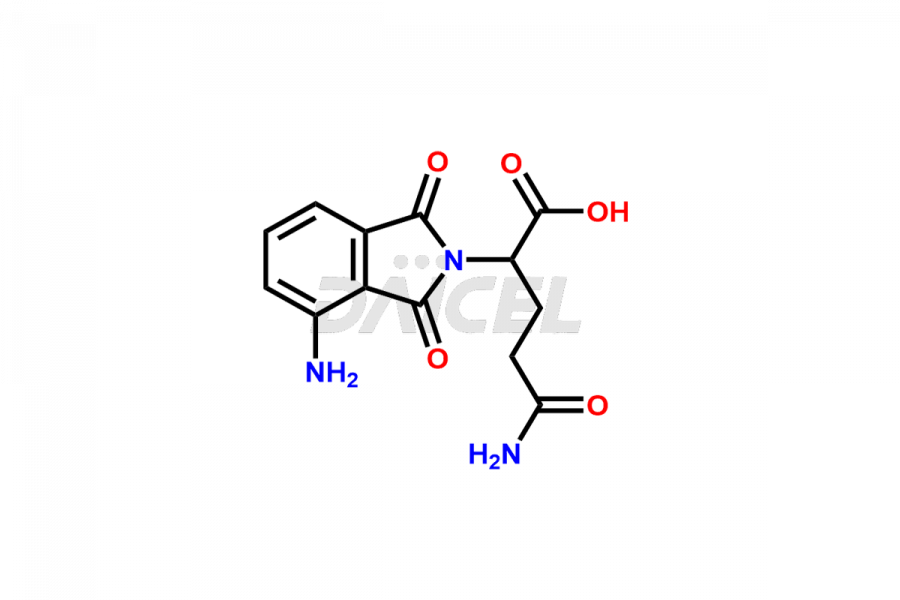
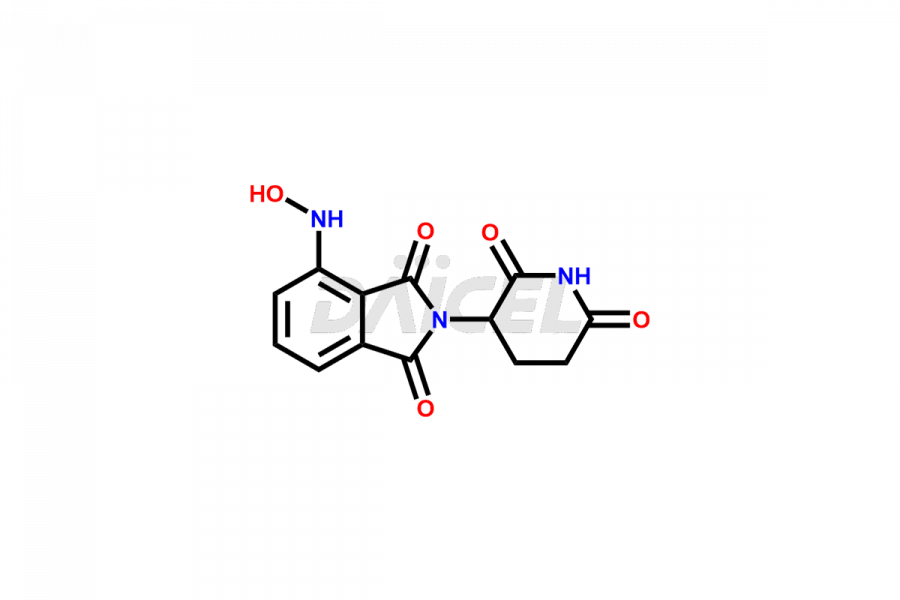
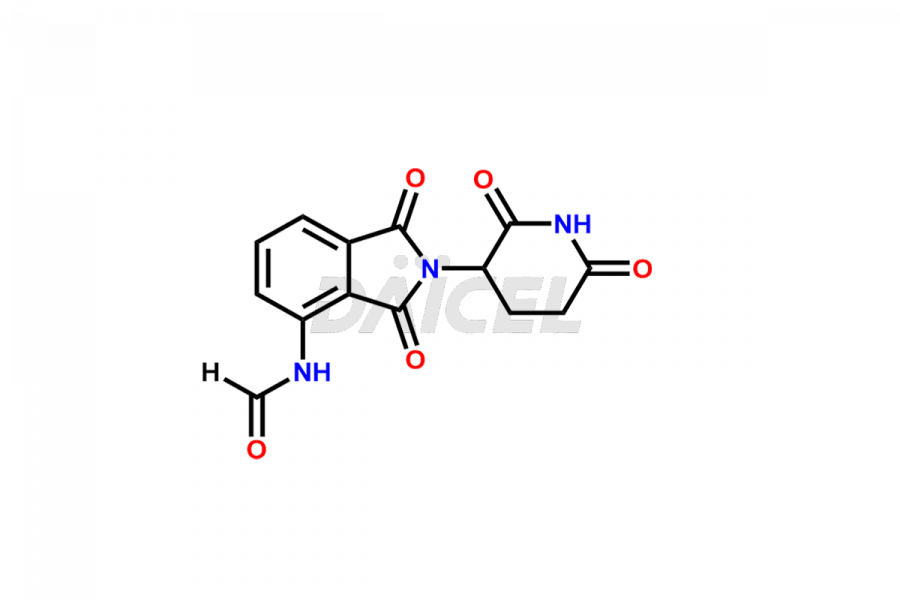
Daicel Pharma offers Pomalidomide impurity standards, including Pomalidomide formyl impurity, POM Hydrolysis Impurity 1, and POM Hydrolysis Impurity 2. Their presence can affect the effectiveness, stability, and safety of Pomalidomide. Daicel Pharma synthesizes custom Pomalidomide impurities and delivers them worldwide following the client’s needs.
Pomalidomide [CAS: 19171-19-8] is a thalidomide derivative that treats multiple myeloma in individuals who have not responded to earlier medications. It functions as an antineoplastic, immunomodulator, and angiogenesis inhibitor. It has similar functions to thalidomide.
Pomalidomide: Use and Commercial Availability
Pomalidomide is for individuals diagnosed with multiple myeloma who have undergone two previous treatments. It treats Kaposi’s sarcoma (KS) in AIDS patients who have not responded to highly active antiretroviral therapy (HAART) and HIV-negative patients with KS. Pomalidomide, marketed as Imnovid, has received approval for treating adult patients with multiple myeloma who have undergone a prior therapy regimen involving Lenalidomide. The medication is also available under the brand name Pomalyst.
Pomalidomide Structure and Mechanism of Action
The chemical name of Pomalidomide is 4-Amino-2-(2,6-dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione. Its chemical formula is C13H11N3O4, and its molecular weight is approximately 273.24 g/mol.
Pomalidomide prevents the proliferation of Lenalidomide-resistant multiple myeloma cell lines. It induces apoptosis of hematopoietic tumor cells.
Pomalidomide Impurities and Synthesis
Impurities may form as byproducts or introduce during the synthesis1 of Pomalidomide due to a variety of variables such as reaction conditions, starting materials, and purifying procedures. These impurities can impact the final drug product’s quality, effectiveness, and safety. Related chemicals, leftover starting ingredients, degradation products, and reaction byproducts are some types of common Pomalidomide impurities.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Pomalidomide impurity standards such as Pomalidomide formyl impurity, POM Hydrolysis Impurity 1, and POM Hydrolysis Impurity 2. The Certificate of Analysis (CoA) provides detailed characterization information, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, by request, Daicel Pharma can provide further characterization details like 13C-DEPT. We have the technical capabilities to synthesize Pomalidomide impurities.
References
FAQ's
References
- Muller, George W.; Stirling, David I.; Chen, Roger S. -c, Substituted 2(2,6-Dioxopiperidin-3-yl)Phthalimides And -1-Oxoisoindolines And Method Of Reducing TNF-Alpha Levels, Celgene Corp., United States, WO9803502A1, January 29, 1998
- Shahbazi, Shandiz; Peer, Cody J.; Polizzotto, Mark N.; Uldrick, Thomas S.; Roth, Jeffrey; Wyvill, Kathleen M.; Aleman, Karen; Zeldis, Jerome B.; Yarchoan, Robert; Figg, William D., A sensitive and robust HPLC assay with fluorescence detection for the quantification of Pomalidomide in human plasma for pharmacokinetic analyses, Journal of Pharmaceutical and Biomedical Analysis Volume: 92, Pages: 63-68, 2014
Frequently Asked Questions
How are Pomalidomide impurities form during the synthesis of the drug?
Impurities form as byproducts during the various steps of the synthetic process of Pomalidomide. Factors like reaction conditions, starting materials, and purification methods may cause impurity formation.
What are the acceptable impurity levels in Pomalidomide?
Regulatory bodies set permissible limits for impurities in Pomalidomide based on safety and effectiveness factors. These restrictions are in pharmacopeial monographs or recommendations.
Are Pomalidomide impurities eliminated from the drug?
While attempts may reduce impurities, eliminating them is difficult. Their purpose is to keep them within permissible levels set by regulatory bodies. Strict production practices and quality control techniques help maintain the purity and safety of Pomalidomide.
What are the temperature conditions required to store Pomalidomide impurities?
Pomalidomide impurities should be stored at a controlled room temperature of 2-8°C or as described on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.