PILOCARPINE
General Information
Pilocarpine Impurities and Pilocarpine
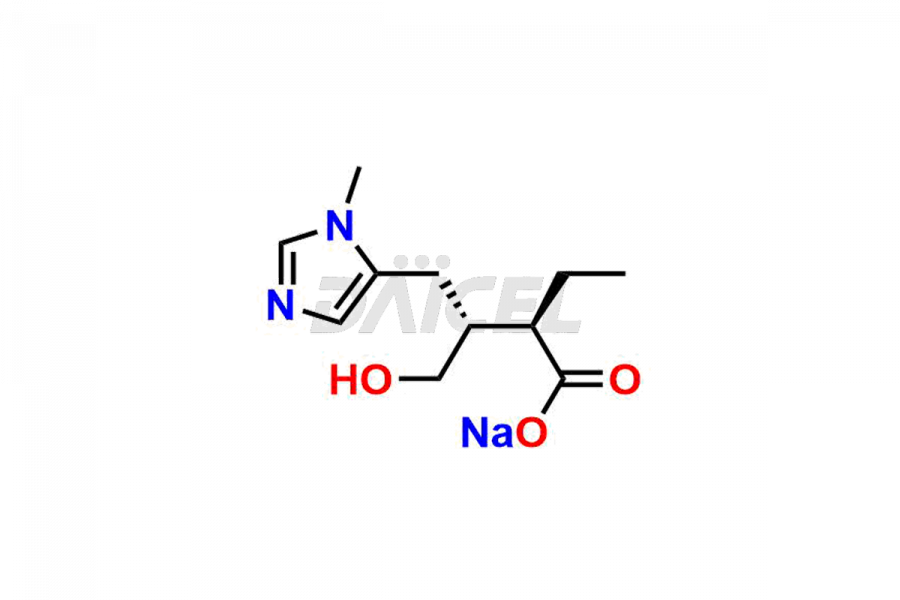
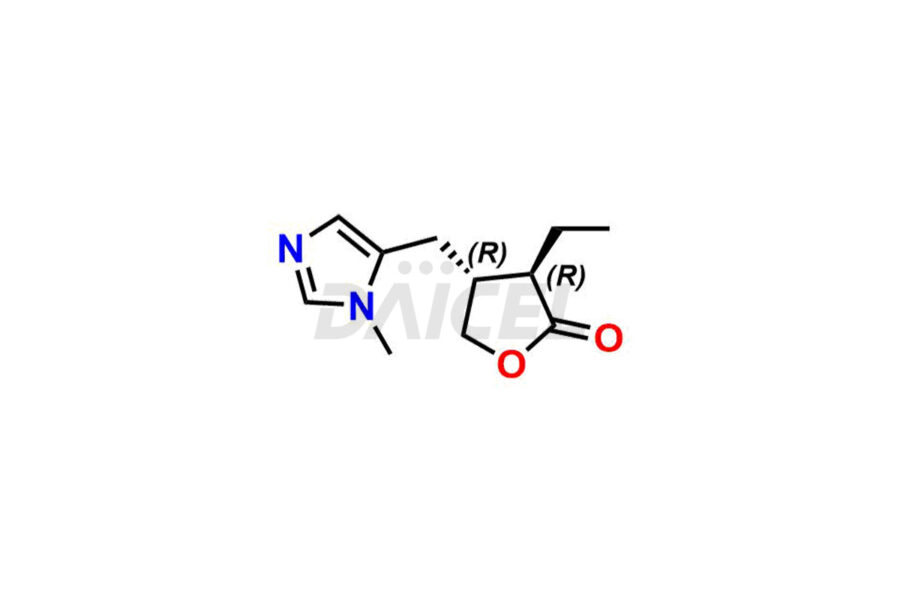
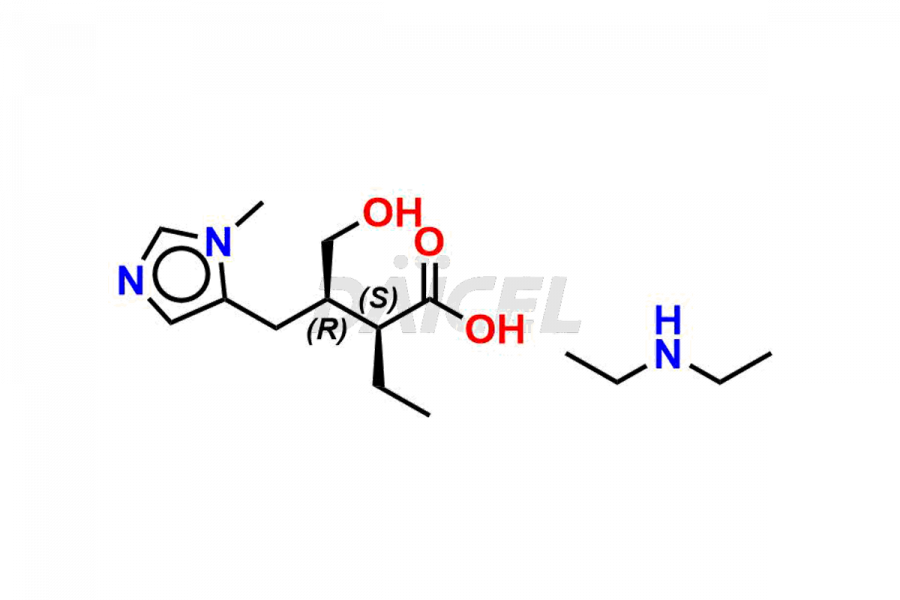
Daicel Pharma prepares Pilocarpine impurity standards, Isopilocarpine, Isopilocarpic acid, and Pilocarpic acid. The impurities can impact Pilocarpine’s efficacy, stability, and safety. Daicel Pharma provides custom synthesis of Pilocarpine impurities and delivers them worldwide.
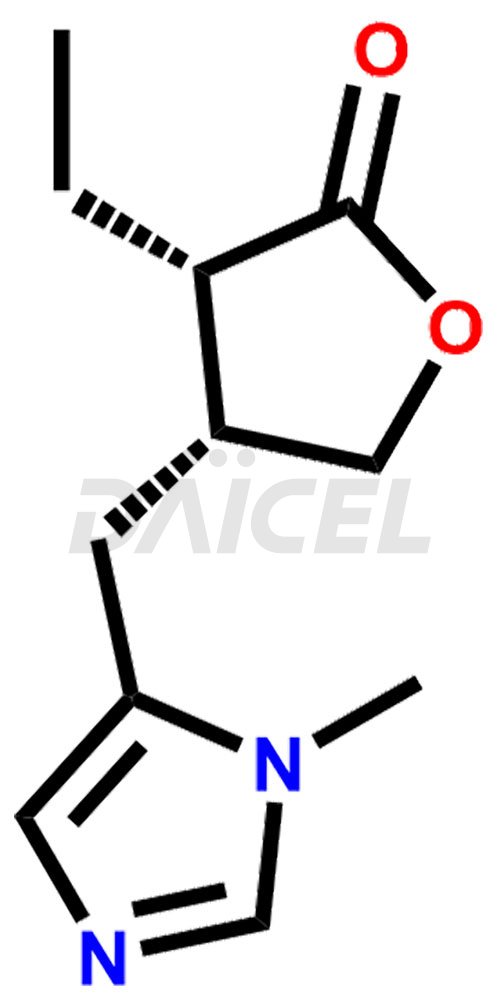
Pilocarpine [CAS: 92-13-7] is a cholinergic receptor agonist and the (-)-enantiomer of pilocarpine. It is an antiglaucoma medication. Pilocarpine acts specifically as a muscarinic agonist, targeting cholinergic muscarinic receptors. It does not affect nicotinic receptors. Pilocarpine reduces pupil size and treats glaucoma.
Pilocarpine: Use and Commercial Availability
Pilocarpine is an oral cholinergic agonist that alleviates dry mouth symptoms in individuals with keratoconjunctivitis sicca (Sjögren syndrome) or xerostomia caused by local irradiation. This medication is available under certain tradenames such as Isopto Carpine, Ocusert Pilo-20, Pilopine HS, Salagen, and Vuity.
Pilocarpine Structure and Mechanism of Action
The chemical name of Pilocarpine is (3S,4R)-3-Ethyldihydro-4-[(1-methyl-1H-imidazol-5-yl)methyl]-2(3H)-furanone. Its chemical formula is C11H16N2O2, and its molecular weight is approximately 208.26 g/mol.
Pilocarpine stimulates muscarinic receptors and smooth muscles such as the iris and secretory glands.
Pilocarpine Impurities and Synthesis
Pilocarpine impurities refer to the unwanted substances or byproducts that may be present in Pilocarpine, a medication used for various purposes. Synthesis1 of Pilocarpine involves the chemical process of preparation of the compound through specific reactions and procedures.
Daicel Pharma offers a Certificate of Analysis (CoA) for Pilocarpine impurity standards, including Isopilocarpine, Isopilocarpic acid, and Pilocarpic acid. Our cGMP-certified analytical facility provides a comprehensive CoA with detailed characterization data like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterizations like 13C-DEPT are available upon request. We offer Pilocarpine impurities and labeled compounds for assessing generic Pilocarpine.
References
FAQ's
References
- Compagnone, Reinaldo S.; Rapoport, Henry, Chirospecific synthesis of (+)-pilocarpine, Journal of Organic Chemistry, Volume: 51, Issue: 10, Pages: 1713-19, 1986
- Dziedzic, Stanley W.; Gitlow, Stanley E., GLC determination of pilocarpine, Journal of Pharmaceutical Sciences, Volume: 65, Issue: 8, Pages: 1262-4, 1976
Frequently Asked Questions
Can Pilocarpine impurities be removed?
It is possible to remove Pilocarpine impurities through appropriate purification methods.
Are there regulatory guidelines for Pilocarpine impurities?
Yes, there are regulatory guidelines in place for Pilocarpine impurities.
What are the temperature conditions required to store Pilocarpine impurities?
Pilocarpine impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
How are impurities detected and analyzed in Pilocarpine?
Various analytical techniques are employed to detect and analyze impurities in Pilocarpine. These methods can include chromatography (such as HPLC), spectroscopy (such as UV-Vis or IR), mass spectrometry, and other validated analytical procedures. These techniques help identify and quantify impurities, enabling manufacturers to assess the purity and quality of the drug.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.