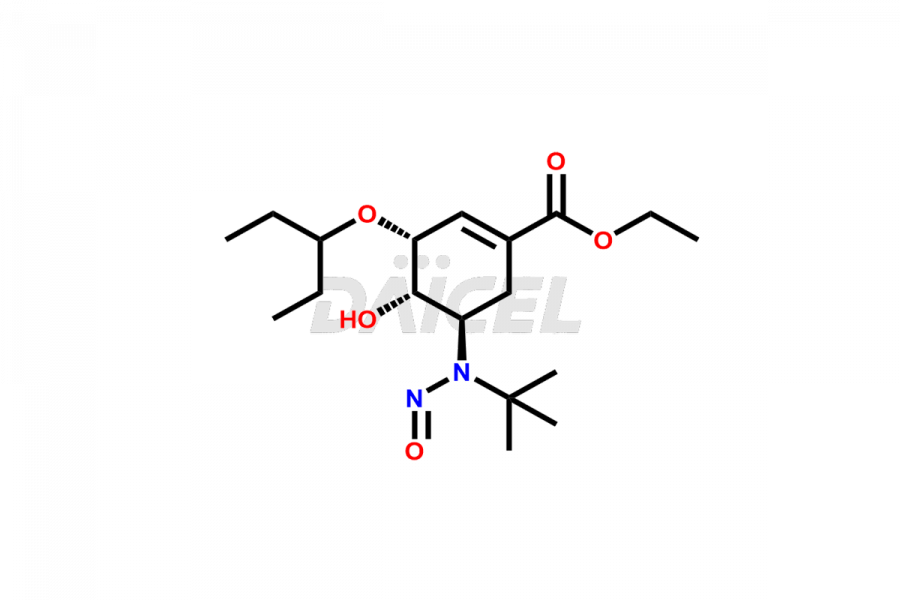
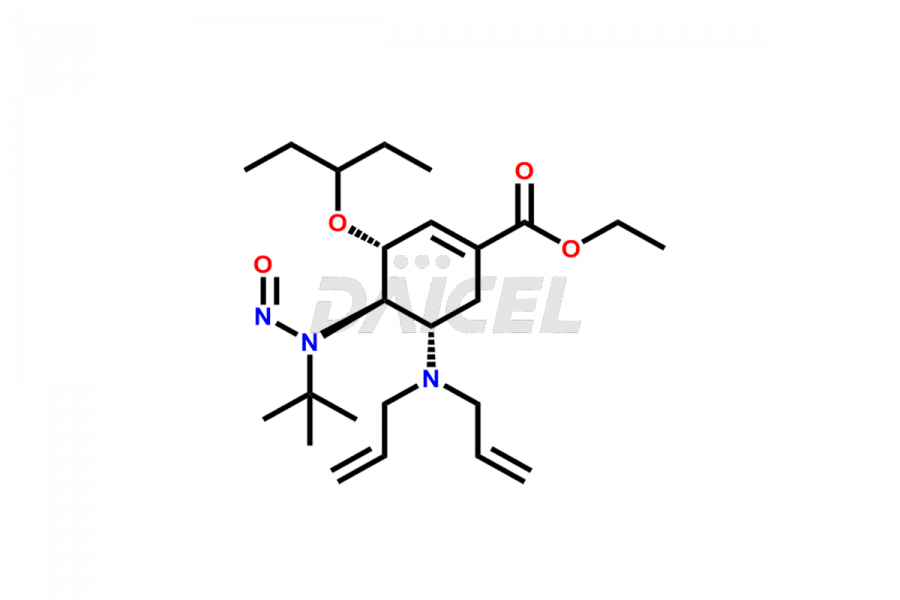
Oseltamivir
LOAD MORE
You're viewed all 38 products
References
FAQ's
References
- Willard Lew, Choung U. Kim, Hongtao LiuMatthew A. Williams, Carbocyclic compounds, Gilead Sciences, Inc, US5866601A, February 2, 1999
- Narasimhan, Balasubramanian; Abida, Khan; Srinivas, Kona, Stability indicating RP-HPLC method development and validation for oseltamivir API, Chemical & Pharmaceutical Bulletin, Volume: 56, Issue: 4, Pages: 413-417, 2008
Frequently Asked Questions
How can degradation impurities be detected in Oseltamivir?
Reverse-phase high-performance liquid chromatography method helps detect and analyze the degradation impurities in Oseltamivir.
How can impurities affect the efficacy of Oseltamivir?
Impurities in Oseltamivir can affect the drug’s efficacy by decreasing its potency or interfering with its mechanism of action.
Which solvent help in the analysis of Oseltamivir impurities?
Acetonitrile or Methanol are the solvents used in analyzing many Oseltamivir impurities.
What are the temperature conditions required to store Oseltamivir impurities?
Oseltamivir impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.