LOAD MORE
You're viewed 9 of 20 products
Daicel Pharma provides high-quality Nilotinib impurities, including Nilotinib Regio isomer (Nilotinib impurity-9), NTB Amine Regio Isomer, 2- Methyl Isomer Impurity of Nilotinib, Nilotinib Impurity E CRS, Nilotinib Impurity F CRS, and so on. These impurities are essential for evaluating Nilotinib quality, stability, and safety. Furthermore, Daicel Pharma provides custom synthesis of Nilotinib impurities and ships them worldwide.
Nilotinib [CAS: 641571-10-0] is a tyrosine kinase inhibitor that treats Chronic Myeloid Leukemia (CML).
Nilotinib treats Chronic Myeloid Leukemia (CML) in patients who have failed or are resistant to other therapies. It is available commercially under the brand name Tasigna. Tasigna is approved to treat adults and children with newly diagnosed Philadelphia Chromosome-Positive Chronic Myelogenous Leukaemia (CML) in the chronic phase.
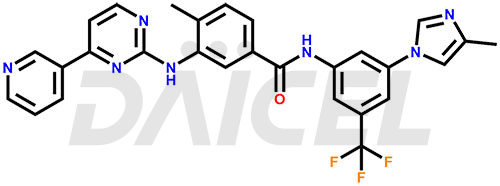
The chemical name of Nilotinib is 4-Methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzamide. Its chemical formula is C28H22F3N7O, and its molecular weight is approximately 529.5 g/mol.
Nilotinib inhibits Bcr-Abl kinase. It stabilizes and binds to the kinase domain of the Abl protein.
Unwanted components or by-products that might be present in the Nilotinib drug are known as impurities. These impurities may develop due to the drug’s synthesis1, storage, or deterioration. Nilotinib’s purity, safety, and effectiveness must be controlled and monitored.
Daicel provides a Certificate of Analysis (CoA) of Nilotinib impurity standards like Nilotinib Regio isomer (Nilotinib impurity-9), NTB Amine Regio Isomer, 2- Methyl Isomer Impurity of Nilotinib, Nilotinib Impurity E CRS, Nilotinib Impurity F CRS, and so on. The CoA is offered from a cGMP-compliant analytical facility and includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. If requested, we give additional characterization data, such as 13C-DEPT. Daicel Pharma can provide unknown Nilotinib impurities or degradation products. Each delivery comes with a complete characterization report.
Nilotinib impurities' control is through strict quality control measures during manufacturing. Regulatory authorities set specific limits for impurity levels that must be adhered to.
Nilotinib impurities can affect its effectiveness by altering the drug's pharmacokinetics, pharmacodynamics, or stability. It is crucial to control and monitor impurities so that the drug maintains its therapeutic potency and delivers the desired therapeutic outcomes.
Nilotinib generics are thoroughly checked for impurities to assure their quality, safety, and efficacy. Comparative studies and thorough quality control help guarantee that generic versions are equal regarding purity and drug efficacy.
Nilotinib impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.