LOAD MORE
You're viewed 9 of 13 products
Daicel Pharma is a trusted provider of quality Molnupiravir impurity standards such as 4-amino-1-((2S,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one, Molnupiravir Anomer, Molnupiravir Enantiomer, Molnupiravir Impurity-F, Molnupiravir Impurity-A, and many more. These impurities are critical in determining Molnupiravir quality, stability, and biological safety. Additionally, Daicel Pharma can synthesize Molnupiravir impurities according to precise customer specifications while guaranteeing worldwide delivery.
Molnupiravir [CAS: 2492423-29-5] is an antiviral drug that treats viral infections such as SARS-CoV-2, the virus caused by COVID-19.
Molnupiravir is an investigative drug that treats severe acute respiratory syndrome (SARS) coronavirus 2 (CoV-2) infection. It has shown activity against several other RNA viruses in preclinical studies, including influenza, MERS-CoV, etc.
There has been no approval for Molnupiravir by the regulatory authorities till now.
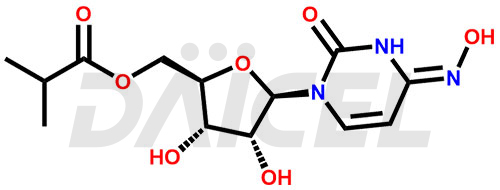
The chemical name of Molnupiravir is (4Z)- 5′-(2-methylpropanoate) 4-oxime Uridine. Its chemical formula is C13H19N3O7, and its molecular weight is approximately 329.31 g/mol.
The precise mechanism of action is under investigation.
Molnupiravir impurities can arise during synthesis due to the storage or use of specific raw materials and intermediates in manufacturing. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Molnupiravir for patient use.
Daicel Pharma provides a comprehensive Certificate of Analysis (CoA) for Molnupiravir impurity standards, such as 4-amino-1-((2S,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one, Molnupiravir Anomer, Molnupiravir Enantiomer, Molnupiravir Impurity-F, Molnupiravir Impurity-A, and many more. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Additionally, upon delivery, a complete 13C-DEPT is also provided. Daicel possesses the technology and expertise to synthesize any unknown Molnupiravir impurity or degradation product.
Methanol and DMSO are the solvents used to analyze many Molnupiravir impurities.
Regulatory authorities provide guidelines for validating analytical methods used for impurity testing in Molnupiravir. These guidelines ensure the accuracy, precision, and reliability of analytical methods.
Molnupiravir impurity levels can vary between manufacturers as each manufacturer uses different processes or starting materials, leading to variations in impurity profiles. Regulatory standards ensure that all impurity levels meet acceptable quality criteria.
Molnupiravir impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.