LOAD MORE
You're viewed 9 of 15 products
For evaluating the purity and safety of Methotrexate, an active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Methotrexate impurity standards. These impurity standards include crucial compounds such as (4-(Methylamino)benzoyl)-D-glutamic acid, Methotrexate Impurity A, Methotrexate Impurity B, Methotrexate Impurity D, and more. Additionally, Daicel Pharma provides worldwide delivery options for Methotrexate impurity standards.
Methotrexate [CAS: 59-05-2] is an antimetabolite and antifolate agent known for its antineoplastic and immunosuppressant properties. It treats acute lymphoblastic leukemia, relapsed or refractory non-Hodgkin lymphoma, psoriasis, and rheumatoid arthritis.
Methotrexate, a potent folic acid antagonist, is an FDA-approved drug that treats rheumatoid arthritis, including juvenile idiopathic arthritis. Methotrexate exhibits anti-inflammatory and immunomodulatory properties. It combines with anti-TNF agents and has demonstrated effectiveness in managing ulcerative colitis, non-Hodgkin’s lymphoma, breast carcinoma, small-cell carcinoma of the lung, head and neck epidermal tumors, and ovarian carcinoma. Methotrexate is available under brand names such as Jylamvo, Otrexup, Otrexup PFS, Rasuvo, and Reditrex.
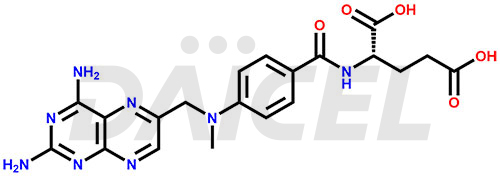
The chemical name of Methotrexate is N-[4-[[(2,4-Diamino-6-pteridinyl)methyl]methylamino]benzoyl]-L-glutamic acid. Its chemical formula is C20H22N8O5, and its molecular weight is approximately 454.4 g/mol.
Methotrexate blocks dihydrofolic acid reductase. It interferes with DNA synthesis, cellular replication, and DNA repair.
Methotrexate1 an antimetabolite and antifolate agent, can potentially contain impurities that may affect its quality and performance. They can include related substances, residual solvents, and degradation products. It is crucial to ensure strict quality control measures during the manufacturing and storage of Methotrexate to minimize impurity levels and maintain its efficacy and safety. Monitoring and controlling impurities in Methotrexate are essential to ensure its therapeutic benefits.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing Methotrexate impurity standards, which include (4-(Methylamino)benzoyl)-D-glutamic acid, Methotrexate Impurity A, Methotrexate Impurity B, Methotrexate Impurity D, and more. In addition, we offer deuterium-labeled Methotrexate standard, Methotrexate-d3, which is essential for conducting bioanalytical research and BA/BE studies. Our Methotrexate impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Methotrexate impurity standards, degradation products, and labeled compounds to evaluate the effectiveness of generic Methotrexate. Each delivery has a comprehensive characterization report.
Yes, specific tests are conducted to identify impurities in Methotrexate. Analytical methods such as high-performance liquid chromatography (HPLC), liquid chromatography (LC), or mass spectrometry (MS) help separate and detect impurities in the drug.
Purification processes can help reduce impurities in Methotrexate. Techniques such as recrystallization, filtration, or chromatography are used during manufacturing to remove impurities and improve the drug's purity.
DMSO is the solvent used when analyzing many impurities in Methotrexate.
Methotrexate impurities should be stored, at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.