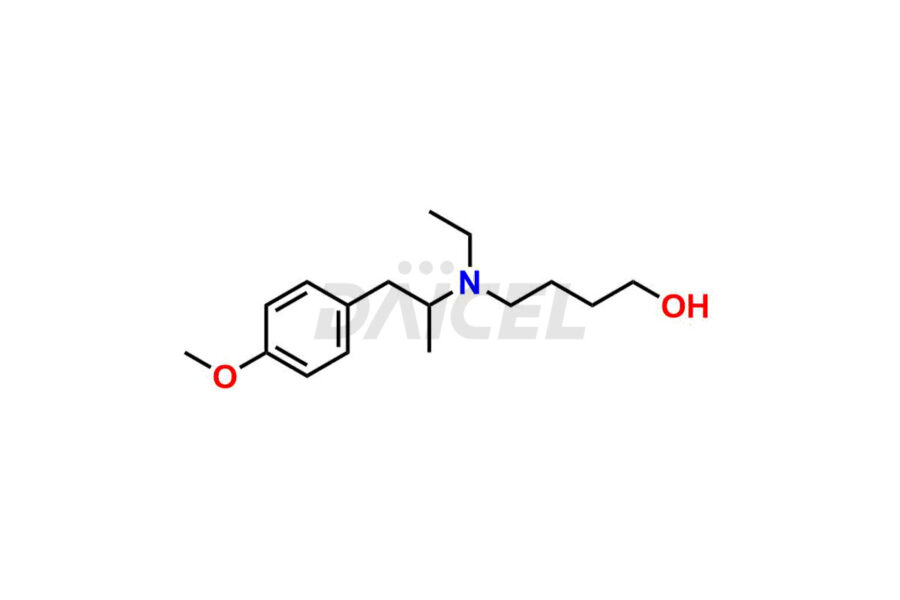
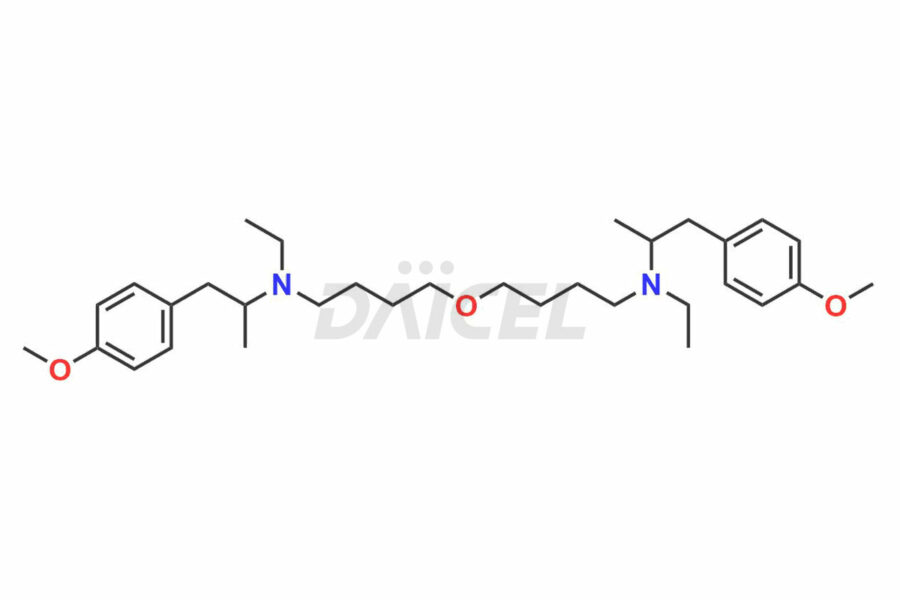
Mebeverine
General Information
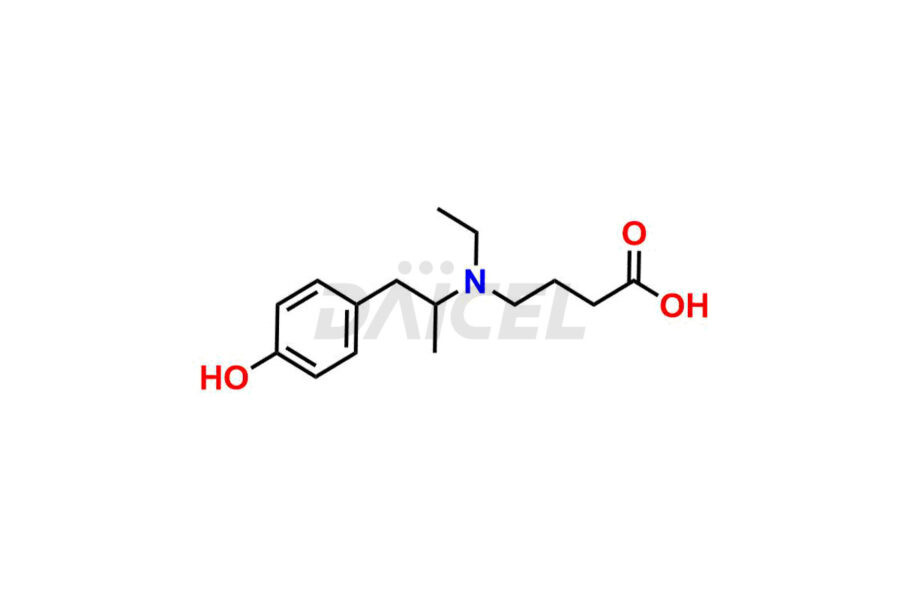
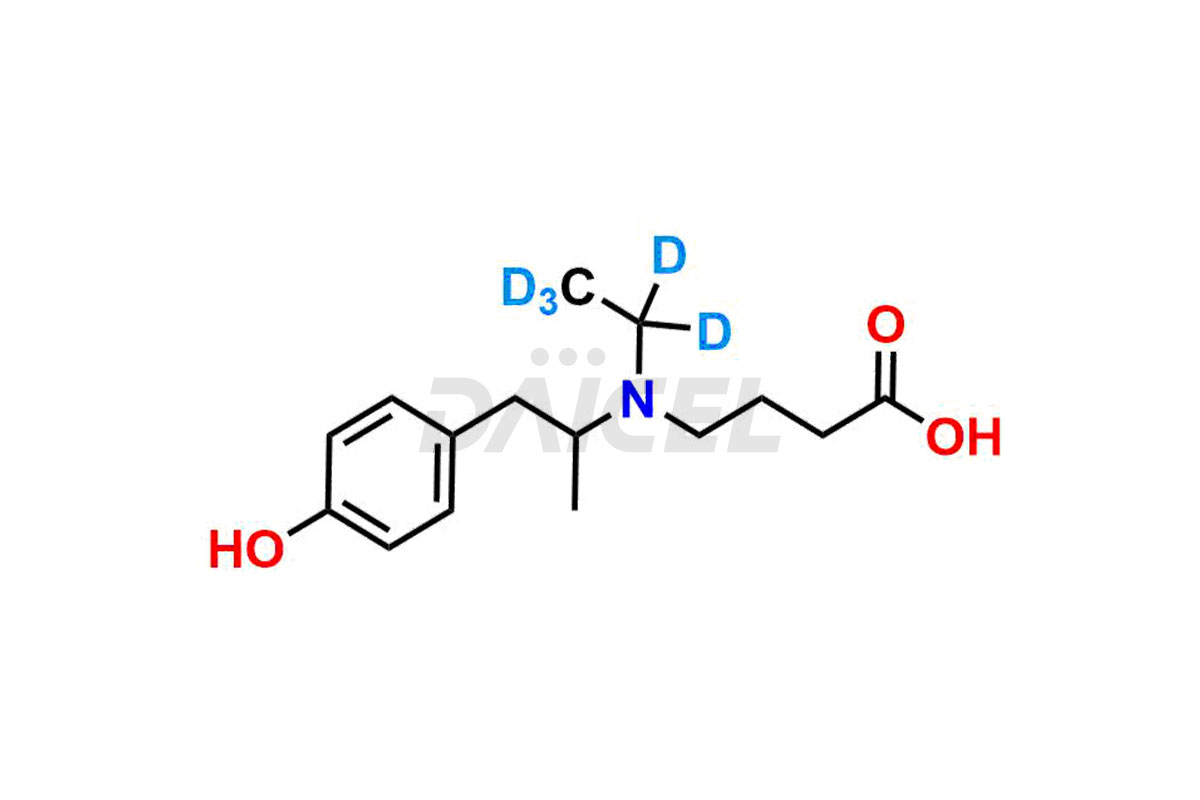
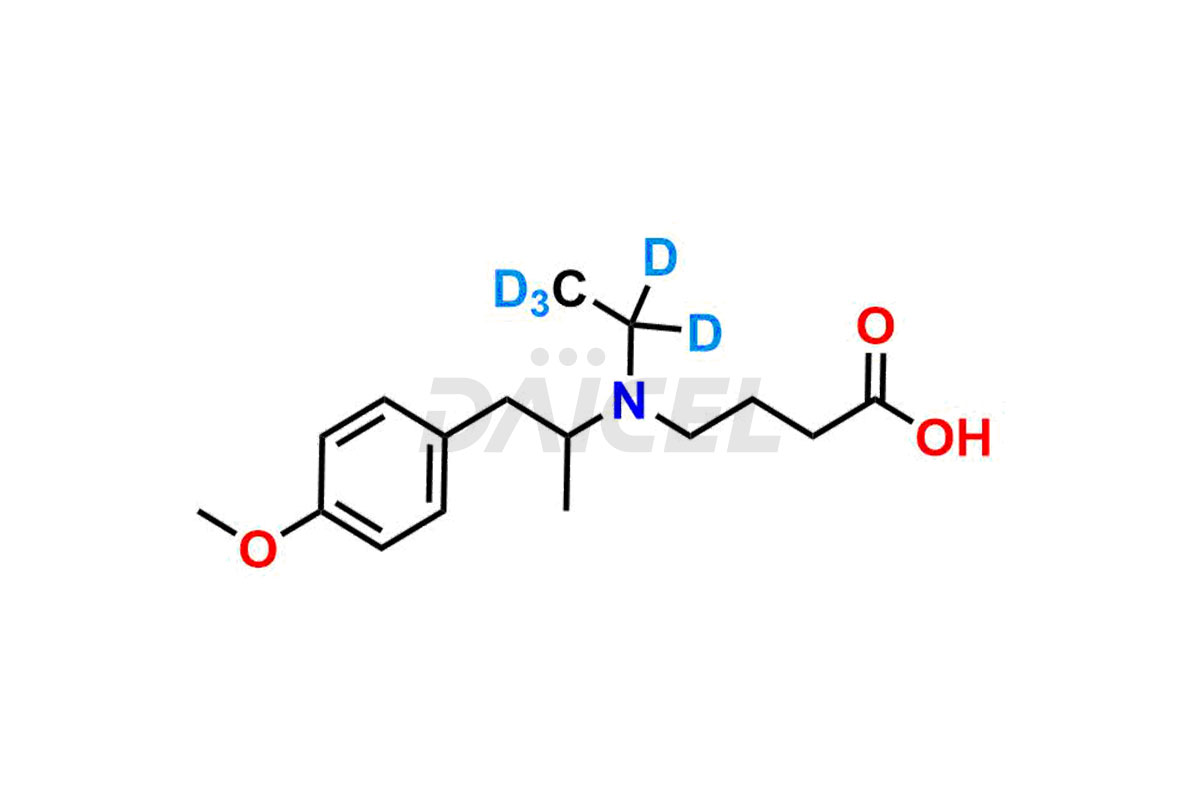
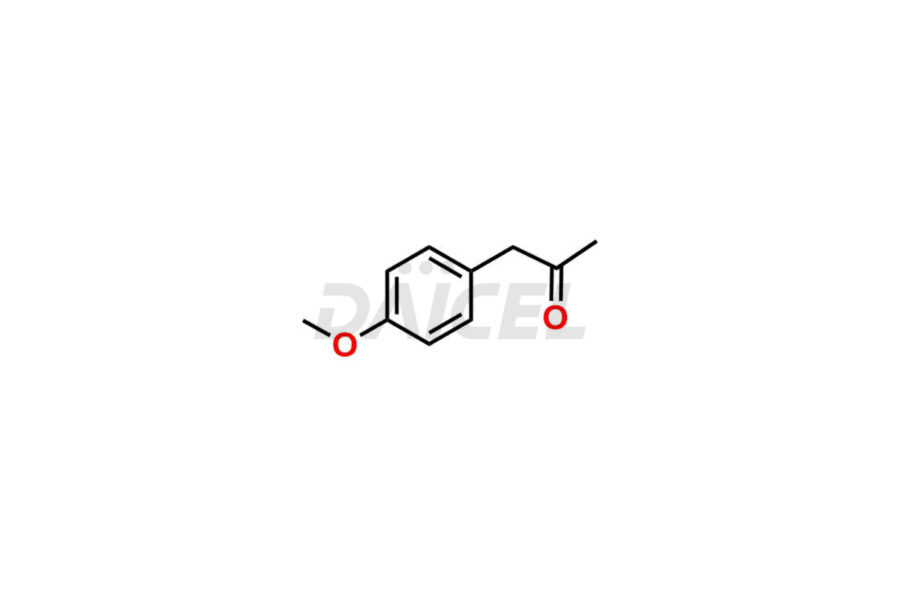
Mebeverine Impurities and Mebeverine
For evaluating the purity and safety of Mebeverine, an active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Mebeverine impurity standards. These impurity standards include crucial compounds such as Desmethyl Mebeverine acid, Mebeverine EP Impurity A, Mebeverine EP Impurity B, Mebeverine EP Impurity C, and Mebeverine impurity J. Additionally, Daicel Pharma provides worldwide delivery options for Mebeverine impurity standards.
Investigation into the therapeutic applications of Mebeverine [CAS: 3625-06-7] has focused on treating irritable bowel syndrome (IBS) and Post-cholecystectomy Gastrointestinal Spasms. This antispasmodic agent is for the symptomatic relief of abdominal pain caused by intestinal smooth muscle spasms and functional disorders due to IBS.
Mebeverine: Use and Commercial Availability
The brand names under which Mebeverine is available are Colofac, Colofac IBS, Aurobeverine, etc. Mebeverine acts as an antispasmodic agent, targeting intestinal smooth muscles directly. Mebeverine therapy has shown positive outcomes in reducing different intestinal symptoms associated with conditions like Irritable Bowel Syndrome (IBS), including abdominal pain, discomfort, distension, irregular bowel habits, and bloating.
Mebeverine Structure and Mechanism of Action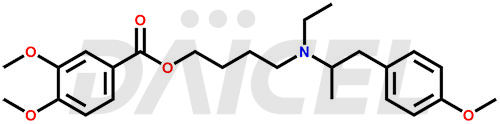
The chemical name of Mebeverine is 3,4-Dimethoxybenzoic acid 4-[ethyl(p-methoxy-α-methylphenethyl)amino]butyl ester. Its chemical formula is C25H35NO5, and its molecular weight is approximately 429.5 g/mol.
The mechanism of action of Mebeverine is not known.
Mebeverine Impurities and Synthesis
To maintain the purity and quality of Mebeverine, regular testing and monitoring of impurities are crucial. Mebeverine, used for gastrointestinal disorder treatment, can contain impurities originating from manufacturing1 or degradation. They may include related substances, isomers, or residual solvents used during production. Compliance with regulatory standards ensures acceptable impurity levels in Mebeverine formulations, ensuring product safety and efficacy. Rigorous quality control measures and analytical techniques help identify and quantify impurities in Mebeverine samples. Monitoring Mebeverine impurities is essential for its effectiveness and quality assurance throughout its shelf life.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for the preparation of Mebeverine impurity standards, which include Desmethyl Mebeverine acid, Mebeverine EP Impurity A, Mebeverine EP Impurity B, Mebeverine EP Impurity C, and Mebeverine impurity J. In addition, we offer Desmethyl Mebeverine acid D5 and Mebeverine acid D5, deuterium-labeled Mebeverine compounds, which are essential for conducting bioanalytical research and BA/BE studies. Our Mebeverine impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Mebeverine impurity standards, degradation products, and labeled compounds to evaluate the effectiveness of generic Mebeverine. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- V. Philips' Gloeilampenfabrieken, Improvements in or relating to the production of esterified amino-alcohols, GB1009082A, November 3, 1965
- De Schutter, J. A.; De Croo, F.; Van der Weken, G.; Van den Bossche, W.; De Moerloose, P., Stability study and quantitative determination of mebeverine hydrochloride in tablets by means of reversed-phase high-performance liquid chromatography, Chromatographia, Volume: 20, Issue: 3, Pages: 185-92, 1985
Frequently Asked Questions
Can Mebeverine impurities affect the bioavailability of the active ingredient?
Some impurities in Mebeverine formulations can potentially impact the bioavailability of the active ingredient, leading to variations in drug absorption and effectiveness.
Are Mebeverine impurities tested for their potential interactions with other drugs?
Impurities in Mebeverine are not tested for specific interactions with other drugs. However, drug-drug interactions involving the active ingredient are considered based on clinical knowledge.
Can Mebeverine impurities be present in different polymorphic forms?
Impurities in Mebeverine can exist in different polymorphic forms, influencing their physicochemical properties and potential impact on product quality.
What is the recommended storage temperature for Mebeverine impurities?
Mebeverine impurities should be stored, at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.