Levodopa
General Information
Levodopa Impurities and Levodopa
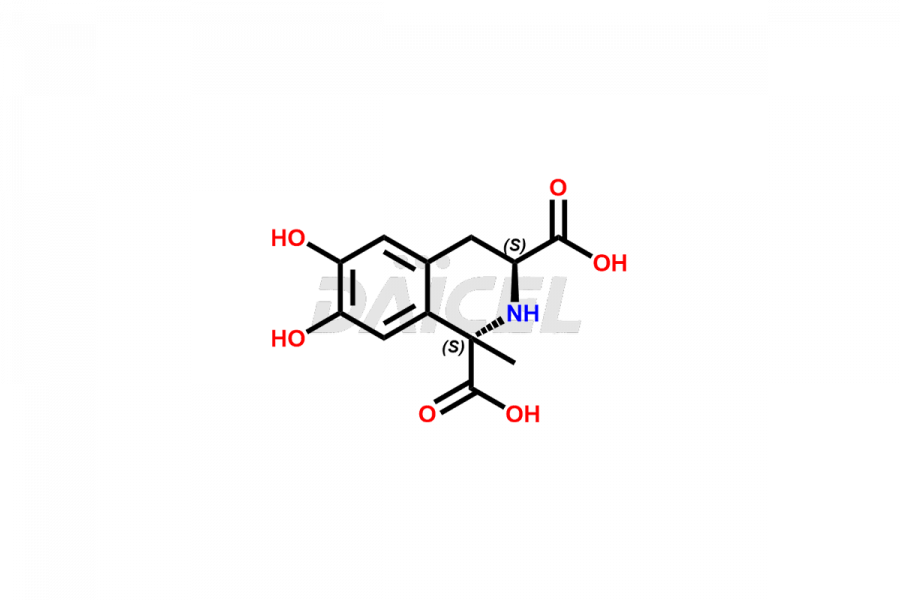
Daicel Pharma offers the best-quality Levodopa impurities, such as 3-(4-hydroxy-3-methoxyphenyl)-2-methyllactic acid, Levodopa Impurity 1513-IP-1, Levodopa Impurity 1513-IP-2, and Tyrosine 3-Methoxy Derivative. They are vital for evaluating Levodopa quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Levodopa impurities and ensures their worldwide delivery.
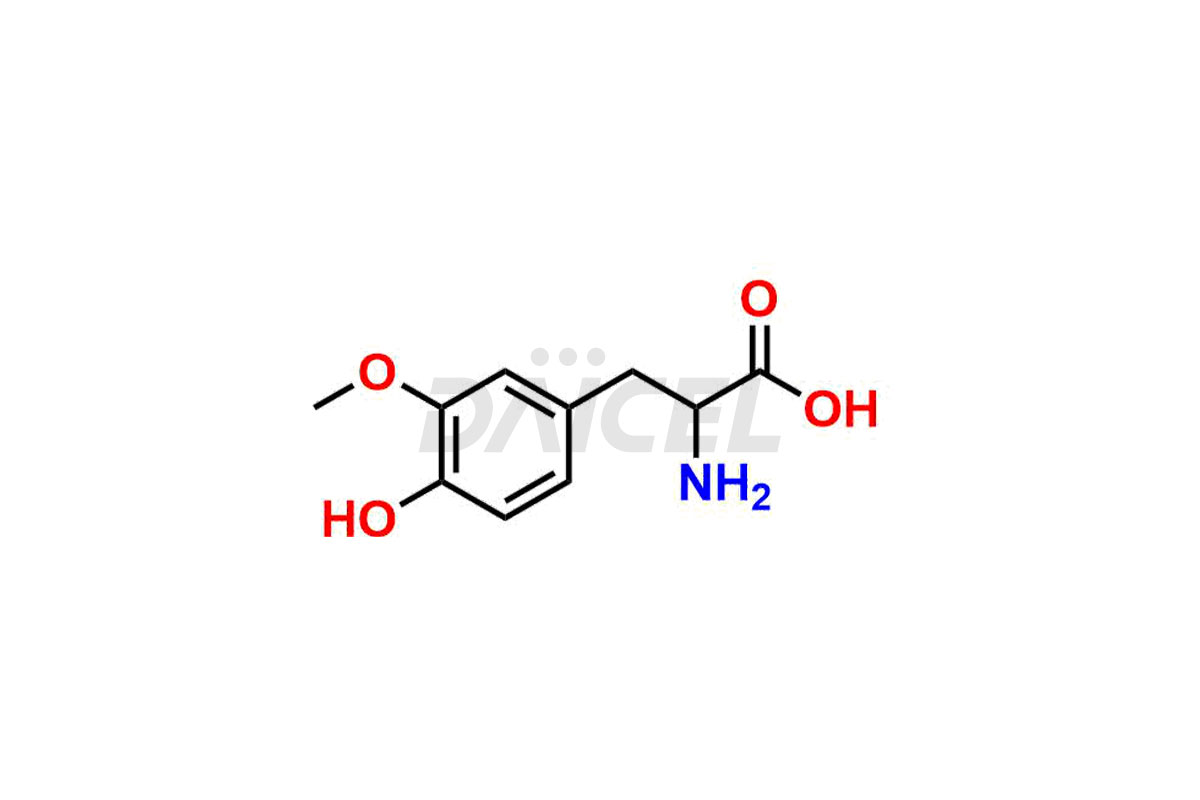
Levodopa [CAS: 59-92-7] or L-Dopa, is a prodrug of dopamine. It is an antiparkinson drug and a dopaminergic agent. It is an L-tyrosine derivative that improves the lives of patients with Parkinson’s disease. It controls the bradykinetic symptoms associated with Parkinson’s disease.
Levodopa: Use and Commercial Availability
Levodopa treats patients with symptoms of Parkinson’s disease. Stiffness, tremors, and muscle spasms are some symptoms of Parkinson’s disease managed with Levodopa. It also helps in treating patients with restless leg syndrome. Its administration is with dopamine decarboxylate inhibitors such as carbidopa. Levodopa is available as oral formulations. Many generic manufacturers prepare Levodopa. These brands are Bendopa, Dopar, Inbrija, Larodopa, etc.
Levodopa Structure and Mechanism of Action
The chemical name of Levodopa is (2S)-2-Amino-3-(3,4-dihydroxyphenyl)propanoic acid. The chemical formula for Levodopa is C9H11NO4, and its molecular weight is approximately 197.19 g/mol.
Levodopa activates postsynaptic dopaminergic receptors. It counteracts the reduction in endogenous dopamine.
Levodopa Impurities and Synthesis
During Levodopa synthesis1, impurities form that may affect the safety and efficacy of the drug. These impurities form during the manufacturing process, purification, or storage of Levodopa. Therefore, Levodopa impurities must be controlled and monitored throughout the drug’s development.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Levodopa impurities, which includes 3-(4-hydroxy-3-methoxyphenyl)-2-methyllactic acid, Levodopa Impurity 1513-IP-1, Levodopa Impurity 1513-IP-2, and Tyrosine 3-Methoxy Derivative. We provide the CoA from a cGMP-compliant analytical facility. It gives complete characterization data2 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We provide additional analytical data on request. Daicel Pharma can prepare any unidentified Levodopa impurity or degradation product. In addition, Daicel Pharma offers highly purified, stable isotope-labeled standards of Levodopa. We provide a complete characterization report to clients on delivery.
References
FAQ's
References
- Krubiner, Alan M., Preparation of L-DOPA, US3734952A, Dec 27, 1968, Hoffmann-La Roche, F., and Co., A.-G. (https://www.lens.org/lens/search/patent/list?q=US3734952)
- Fahn, Stanley; Prasad, A. L. N.; Delesie, Rita, Reliable and simple method for simultaneous determination of dopa and 3-O-methyldopa in plasma and brain, Analytical Biochemistry, Volume: 46, Issue: 2, Pages: 557-75, 1972 DOI: (10.1016/0003-2697(72)90327-2)
Frequently Asked Questions
What are the analytical methods to separate Levodopa from its impurities?
The rapid capillary electrophoresis (CE) and reverse phase liquid chromatographic method help separate Levodopa from its impurities.
What are the possible reasons for the formation of Levodopa impurities?
Starting materials, reagents, unreacted catalysts during synthesis, and poor storage conditions may form Levodopa impurities.
Why is it necessary to remove Levodopa impurities from the drug?
Levodopa impurities can affect drug safety and quality and pose health risks, so it is necessary to remove them from the drug.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.