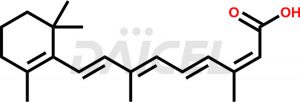
Isotretinoin
General Information
Isotretinoin Impurities and Isotretinoin
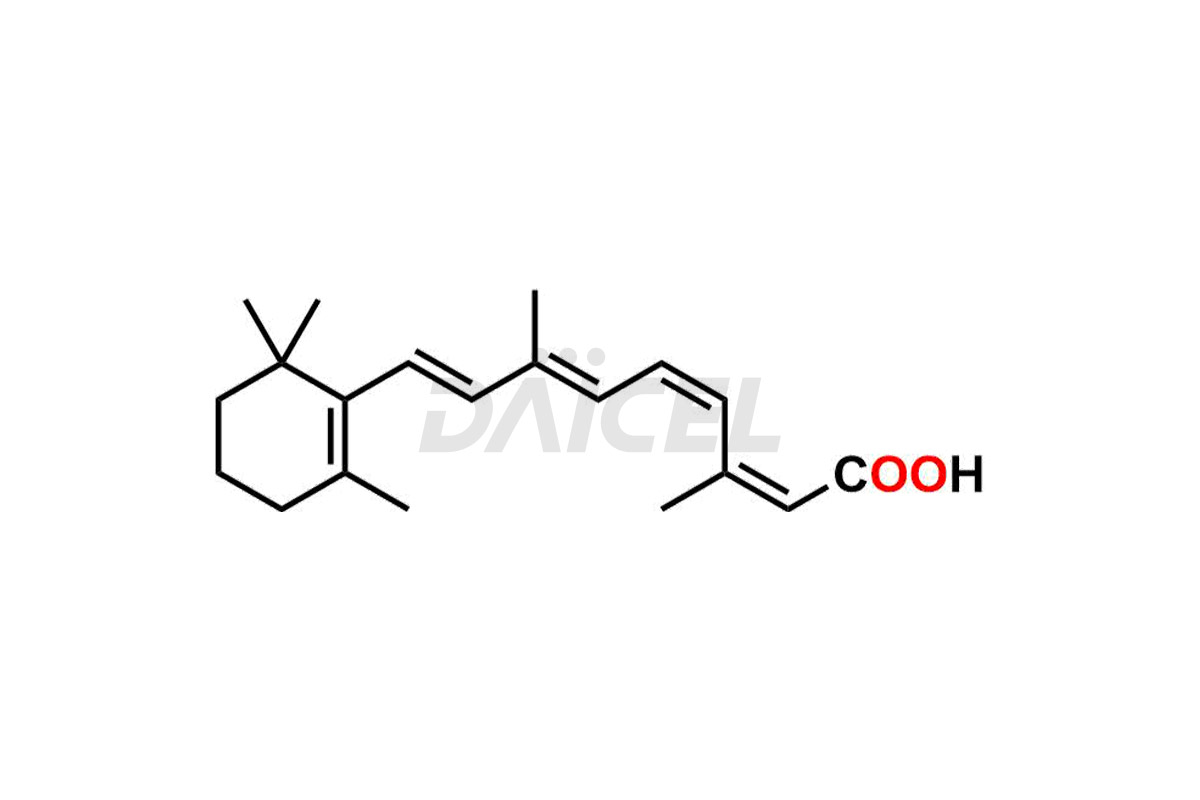
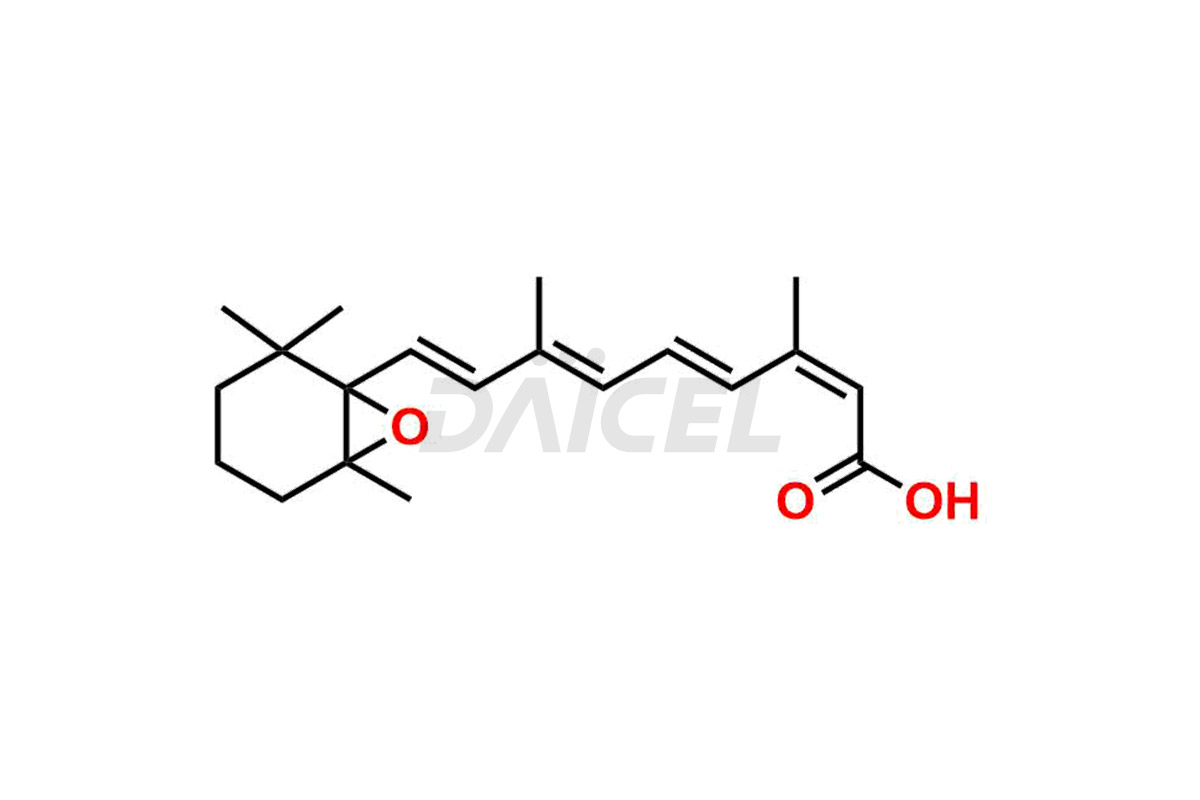
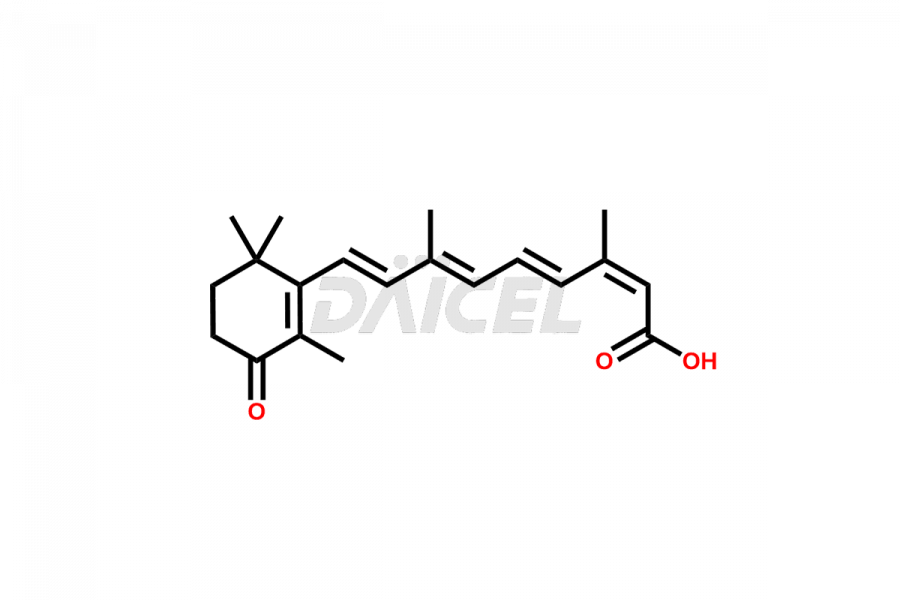
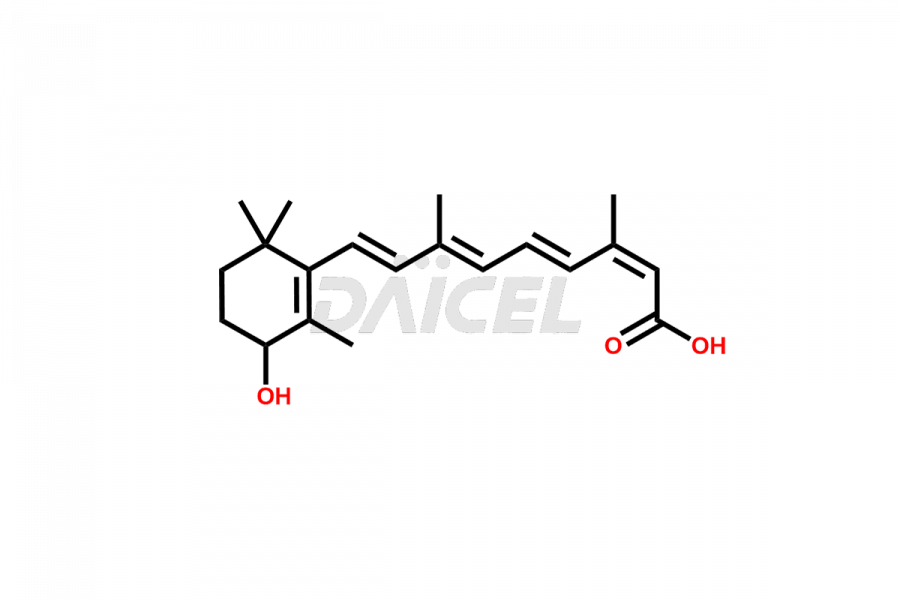
Daicel Pharma synthesizes high-quality Isotretinoin impurities such as 11,13-Di-Cis-Retinoic Acid (Isotretinoin EP Impurity C), 13-cis-5,6-Epoxy-5,6-dihydro retinoic acid, 4-Oxo-13-cis-retinoic acid, and 4-HydroxyIsotretinoin (Isotretinoin Impurity I). The above impurities are crucial in determining the quality, stability, and biological safety of the active pharmaceutical ingredient, Isotretinoin. Moreover, Daicel offers custom synthesis of Isotretinoin impurities and delivers them globally.
Isotretinoin [CAS: 4759-48-2] is 13-cis-retinoic acid. It belongs to the retinoic acid, retinol (vitamin A) family of compounds. It functions as a keratolytic agent, which reduces keratin in the skin and clears pores, making it an effective treatment for severe acne and other skin conditions. The conjugate acid of Isotretinoin is referred to as 13-cis-retinoate.
Isotretinoin: Use and Commercial Availability
Isotretinoin is a retinoid derived from vitamin A used to treat severe acne that has not responded to other treatments like oral and topical antibiotics and topical retinoids. It helps in treating nodulocystic acne, acne conglobata, and scarring acne. Isotretinoin is available as oral capsules under various brand names, like Absorica, Claravis, Myorisan, Accutane, etc.
Isotretinoin Structure and Mechanism of Action
The chemical formula for Isotretinoin is C20H28O2, and its molecular weight is approximately 300.4 g/mol.
The stereoisomer of all-trans retinoic acid (tretinoin) is Isotretinoin. Though the exact mechanism of action is unknown, Isotretinoin helps to suppress sebaceous gland activity.
Isotretinoin Impurities and Synthesis
Isotretinoin impurities like heavy metal and oxidized impurities form during the purification process1. In addition, Isotretinoin impurities also occur from solvents or residual reagents used in the manufacturing process2. Identifying and eliminating such impurities in Isotretinoin is crucial to improve the quality of the drug.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Isotretinoin impurity standards like 11,13-Di-Cis-Retinoic acid (Isotretinoin EP Impurity C), 13-cis-5,6-Epoxy-5,6-dihydro retinoic acid, 4-Oxo-13-cis-retinoic acid, and 4-HydroxyIsotretinoin (Isotretinoin Impurity I). The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity3. We also provide 13C-DEPT and CHN on request. We also give a complete characterization report on delivery.
Daicel has the technology and expertise to prepare unknown Isotretinoin impurities or degradation products. In addition, Daicel provides labeled compounds to quantify the efficacy of generic Isotretinoin. Daicel offers highly pure isotope-labeled standards of Isotretinoin for bioanalytical research and BA/BE studies with the percentage of isotope data in CoA.
References
FAQ's
References
- Ashok Kumar, Dharmendra Singh, Ganesh Devidas Mahale, Ragnesh Kumar Rana, Mahesh Kharade, “A process for preparation of highly pure isotretinoin” WO2008102375A2, August, 28, 2008
- Garbers, C. F.; Shneider, D. F.; Van der Merwe, J. P., “Synthesis of cis-2,cis-4-vitamin A acid by a Wittig condensation”, Journal of the Chemical Society [Section] C: Organic, Issue: 16, Pages: 1982-3, 1968,
- Frolik, Charles A.; Tavela, Thomas E.; Peck, Gary L.; Sporn, Michael B., “High-pressure liquid chromatographic determination of 13-cis-retinoic acid and all-trans-retinoic acid in human plasma” Analytical Biochemistry, Volume: 86, Issue: 2, Pages: 743-50, 1978,
Frequently Asked Questions
How are Isotretinoin impurities formed during its synthesis?
Isotretinoin impurities may form from the by-products of chemical reactions during manufacturing or from the raw materials used to make Isotretinoin.
Do Isotretinoin impurities formed during its synthesis affect its potency?
The impurities formed during Isotretinoin synthesis affect its potency and may also be the reason for the side effects or toxicity of the drug.
What analytical techniques are used to detect Isotretinoin impurities?
Analytical techniques such as HPLC and LC-MS help detect and quantify Isotretinoin impurities.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.