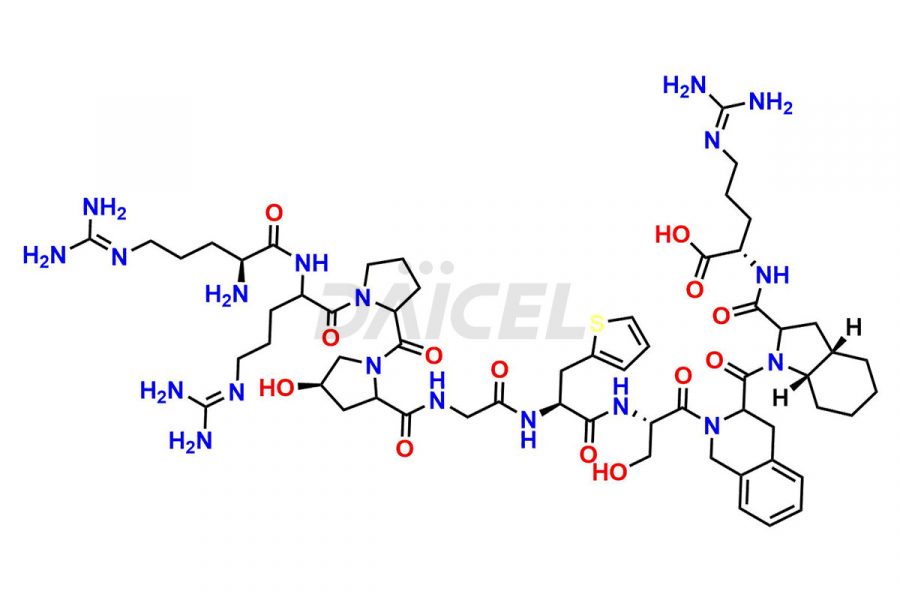
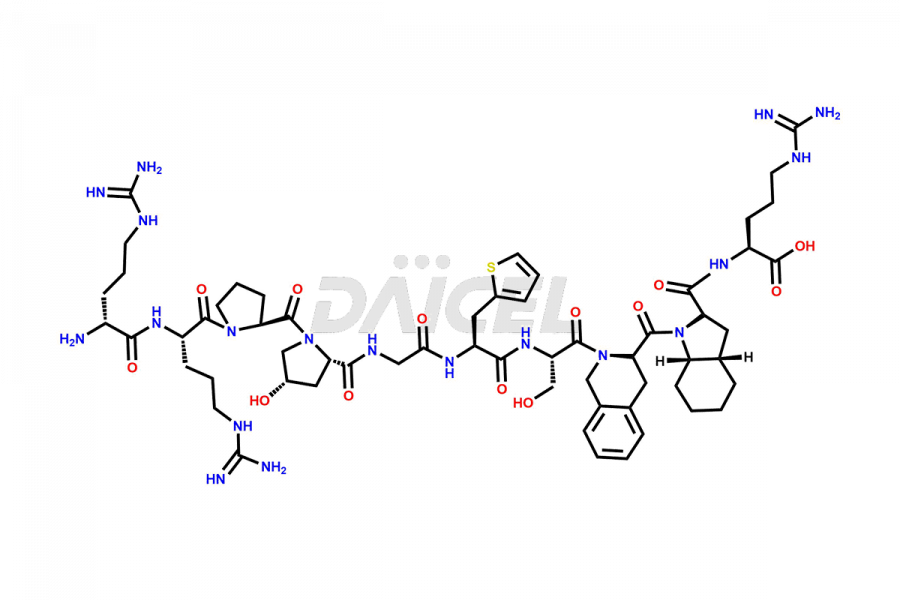
Icatibant
References
- Stephan Henke; Hiristo Anagnostopulos; Gerhard Breipohl; Jochen Knolle; Jens Stechl,; Bemward Scholkens; Hans-Wolfram Fehlhaber; Hermann Gerhards; Franz Hock; Hoechst Aktiengesellschaf, “Peptides Having Bradykevin Antagonist Action” US patent US5648333, July 15, 1997
- Gupta, Praveer; Siriki, Yernaidu; Sadhanala, Trimurthulu; Akula, Ravi Kumar; Alembic Pharmaceuticals Limited, “An improved process for preparation of icatibant by solid phase peptide synthesis and its acetate salt” Indian publication, IN201621021157, June 21, 2016
- Lajin, Bassam; Steiner, Oliver; Fasshold, Lisa; Zangger, Klaus; Goessler, Walter, “The identification and chromatographic separation of a new highly analogous impurity of the active pharmaceutical ingredient icatibant”, European Journal of Pharmaceutical Sciences, Volume: 132, Pages: 121-124, 2019
Frequently Asked Questions
What are the acceptable limits for related peptide Icatibant impurities?
The acceptable limit for related peptide Icatibant impurities is typically 0.5% or less.
How are related peptide Icatibant impurities synthesized?
Related peptide Icatibant impurities are synthesized using the same methods and starting materials as Icatibant itself. Further, they are separated from the desired product during the purification process.
What are the common methods used to synthesize organic Icatibant impurities?
Organic Icatibant impurities form through various chemical reactions, including oxidation, reduction, and cyclization. The methods used to synthesize these impurities will depend on the specific, targeted impurity.
What analytical methods are used to monitor impurities during the synthesis of Icatibant?
Analytical methods such as high-performance liquid chromatography3 (HPLC), and liquid chromatography mass spectrometry (LC-MS) are commonly used to monitor impurities during the synthesis of Icatibant. These methods allow for the identification and quantification of impurities at various stages of the synthetic process.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.