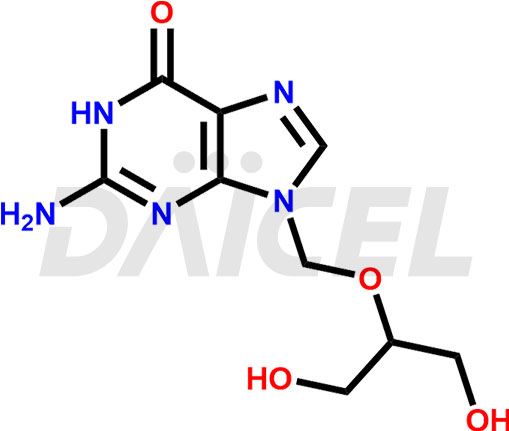
Ganciclovir
General Information
Ganciclovir Impurities and Ganciclovir
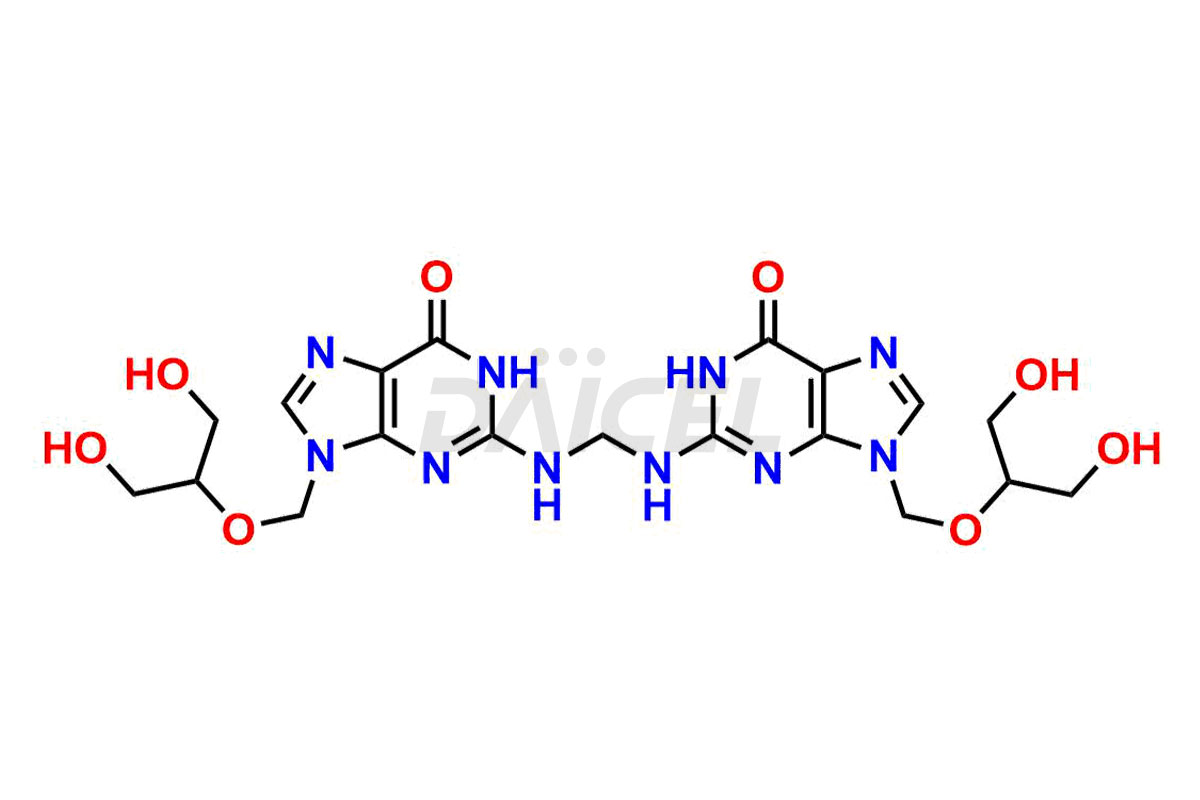
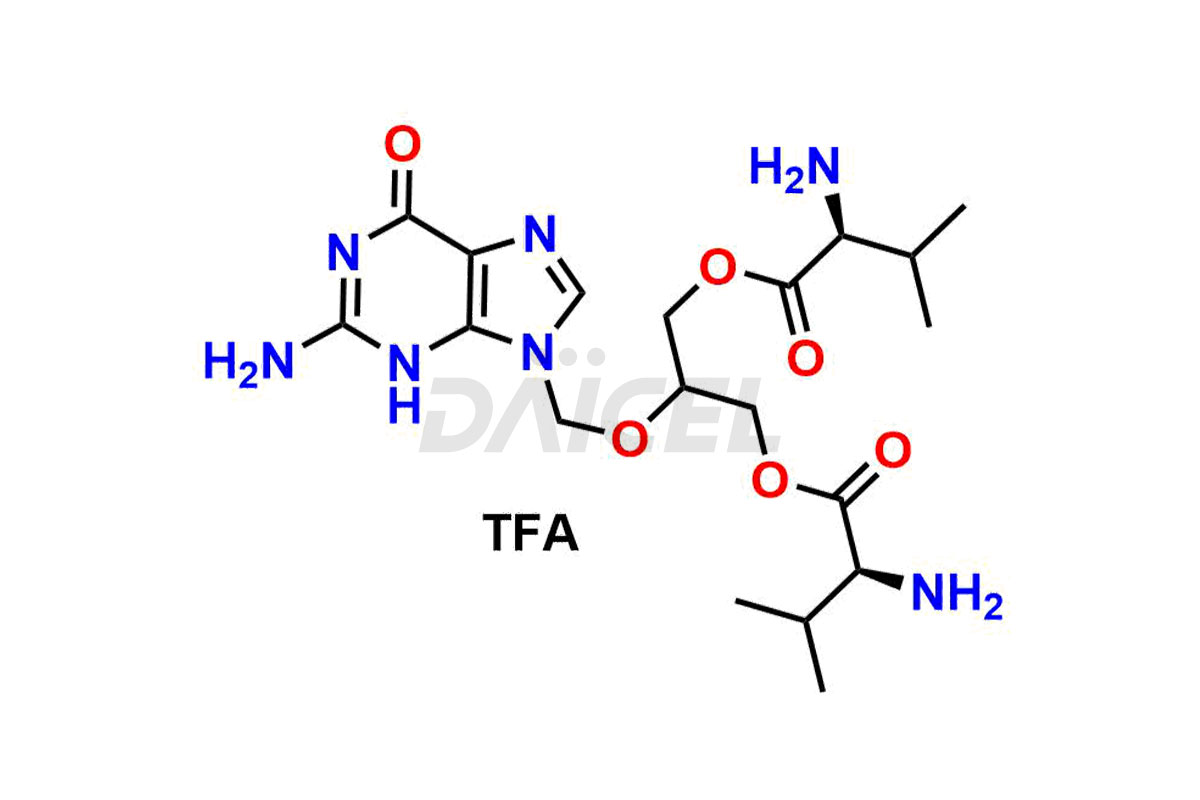
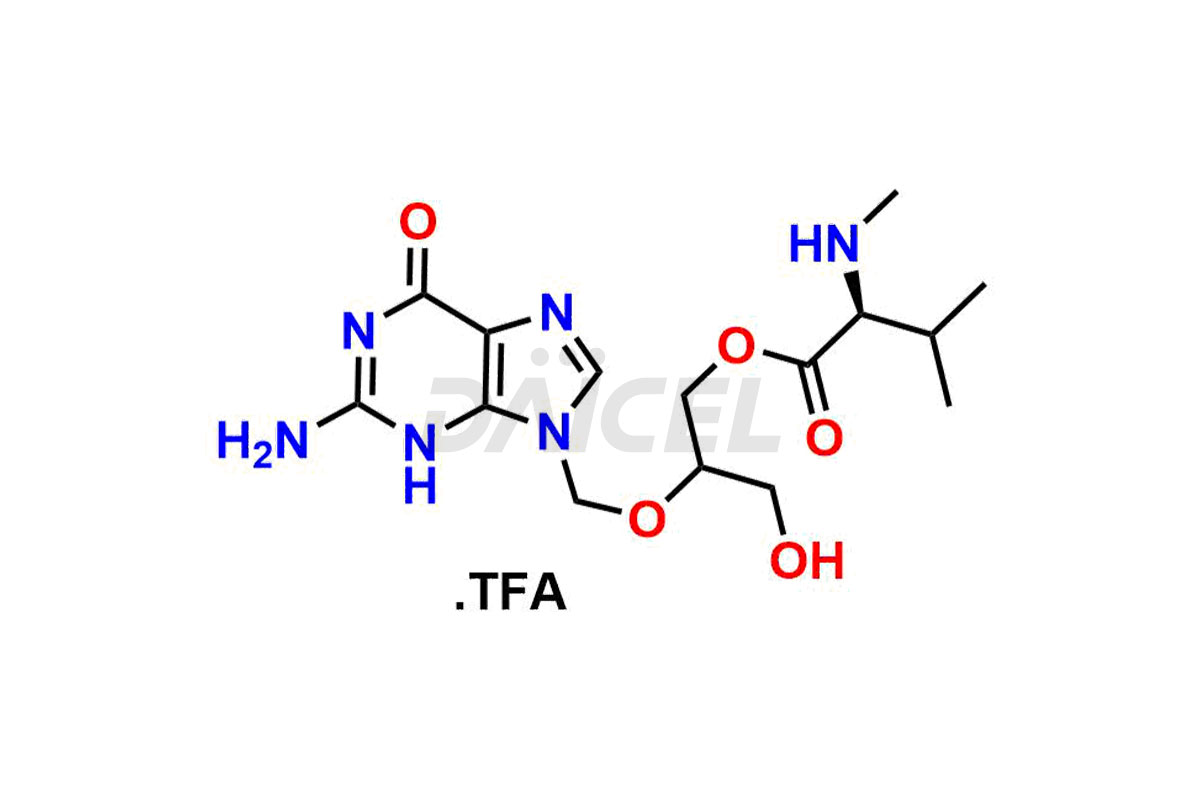
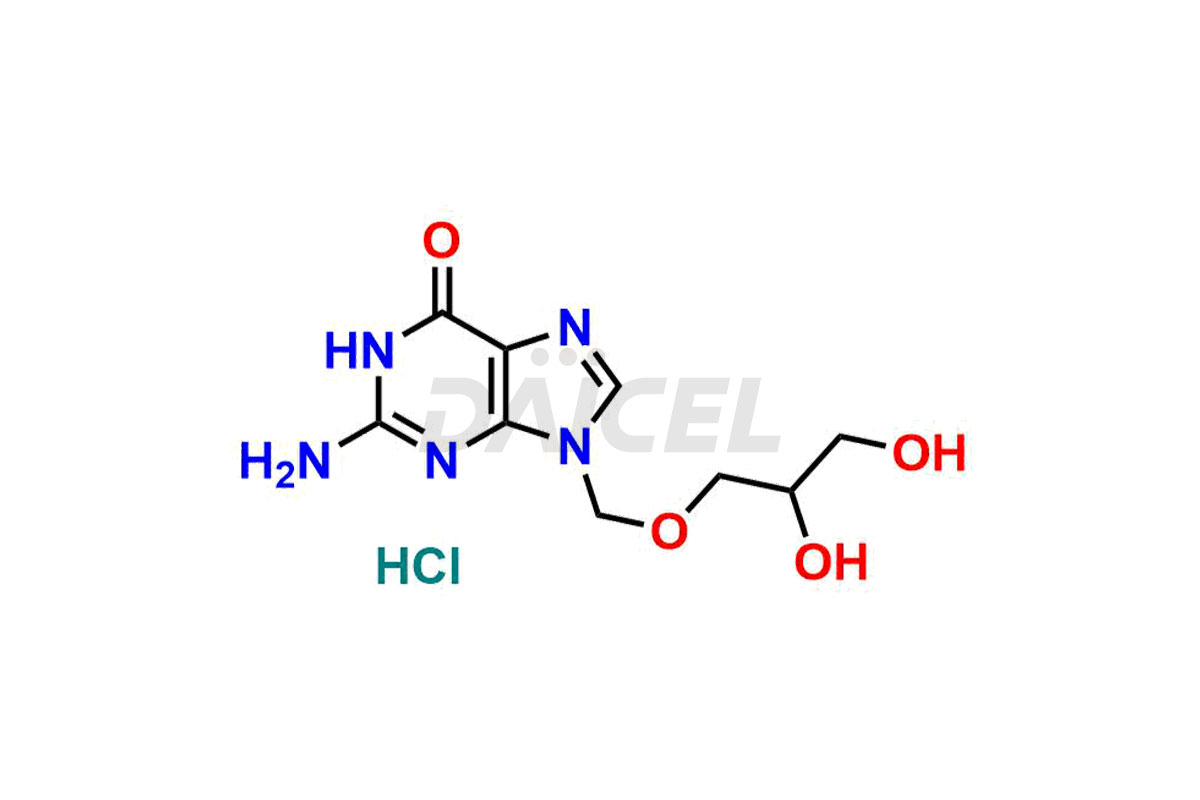
Daicel Pharma synthesizes high-quality Ganciclovir impurities like Ganciclovir dimer, Ganciclovir Divalinate, Ganciclovir Mono N-Methyl Valinate (Mixture of Diastereomers), and Ganciclovir related compound A, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Ganciclovir. Moreover, Daicel Pharma offers custom synthesis of Ganciclovir impurities and delivers them globally.
Ganciclovir [CAS: 82410-32-0] is a synthetic guanine derivative with antiviral properties. It manages cytomegalovirus infections in people with AIDS. This drug functions as both an antiviral agent and an anti-infective medication.
Ganciclovir: Use and Commercial Availability
Ganciclovir is used to manage cytomegalovirus (CMV) infections, which affect the eyes, colon, and esophagus in patients with AIDS or weakened immune systems. It is to prevent and treat CMV infections in patients who have received transplants. The drug is taken orally or administered intravenously. It is available under various brand names, including Cytovene, Ganzyk-Rtu, Ganciclovir Sodium, Vitrasert, and Zirgan.
Ganciclovir Structure and Mechanism of Action 
The chemical name of Ganciclovir is 2-Amino-1,9-dihydro-9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]-6H-purin-6-one. Its chemical formula is C9H13N5O4, and its molecular weight is approximately 255.23 g/mol.
Ganciclovir is an antiviral drug and a synthetic analog of 2’-deoxyguanosine. It inhibits the replication of human cytomegalovirus (CMV).
Ganciclovir Impurities and Synthesis
Ganciclovir impurities are unwanted substances that form during manufacturing1,2 or storage of Ganciclovir drug substance or product. These impurities can affect the drug’s quality, safety, and efficacy. Understanding the formation and synthesis of these impurities is essential to identify and controlling them and ensuring that the drug is of high quality and purity.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Ganciclovir impurity standards, Ganciclovir dimer, Ganciclovir Divalinate, Ganciclovir Mono N-Methyl Valinate (Mixture of Diastereomers), and Ganciclovir related compound A. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity3. We also provide 13C-DEPT and CHN if requested. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Ganciclovir impurity or degradation product.
References
FAQ's
References
- Ogilvie, Kelvin K., Ring open nucleoside analogues, ENS Biologicals Inc US4347360A, August 31, 1982
- Ogilvie, Kelvin K.; Cheriyan, Ukken O.; Radatus, Bruno K.; Smith, Kendall O.; Galloway, Karen S.; Kennell, Wiebke L., Biologically active acyclonucleoside analogs. II. The synthesis of 9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]guanine (BIOLF-62), Canadian Journal of Chemistry, Volume: 60, Issue: 24, Pages: 3005-10, 1982
- Visor, G. C.; Jackson, S. E.; Kenley, R. A.; Lee, G., Reverse-phase HPLC analysis of the antiviral drug, 9-[1,3-dihydroxy-2-propoxy methyl]-guanine (DHPG), with amperometric detection, Journal of Liquid Chromatography, Volume: 8, Issue: 8, Pages: 1475-88, 1985
Frequently Asked Questions
What are the sources of impurities in Ganciclovir?
Impurities in Ganciclovir can originate from starting materials, reagents, or by-products formed during synthesis. They can also occur during the storage or degradation of the drug.
What is the effect of impurities on the efficacy of Ganciclovir?
Ganciclovir impurities reduce the drug efficacy by competing with the active ingredient for receptor binding sites or decreasing the bioavailability of the drug.
Which solvents help in the analysis of Ganciclovir impurities?
Methanol or water are the solvents for analyzing most of the Ganciclovir impurities.
What are the temperature conditions required to store Ganciclovir impurities?
Ganciclovir impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.