LOAD MORE
You're viewed 9 of 13 products
Daicel Pharma specializes in synthesizing impurities for Fingolimod, an active pharmaceutical ingredient. We offer crucial impurities such as 2-Phenethyl Fingolimod Impurity, 3-Phenethyl Fingolimod Impurity, Fingolimod Impurity-1, Fingolimod impurity-3, and Fingolimod Phosphate, which play a vital role in evaluating the purity, and safety of Fingolimod. Daicel Pharma also provides custom synthesis of Fingolimod impurities to meet specific client needs, and we offer worldwide delivery options.
Fingolimod [CAS: 162359-55-9], an orally available immunomodulatory drug, acts as a sphingosine-1-phosphate receptor agonist. It treats relapsing multiple sclerosis, where it functions as an immunosuppressive agent. It also serves as an antineoplastic agent.
Fingolimod, known by its brand name Gilenya, treats relapsing forms of multiple sclerosis, including relapsing-remitting disease, clinically isolated syndrome, and active secondary progressive disease. It reduces inflammation and axonal damage in the central nervous system.
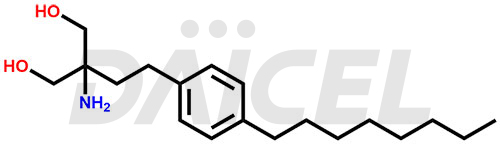
The chemical name of Fingolimod is 2-Amino-2-[2-(4-octyl phenyl)ethyl]-1,3-propanediol. Its chemical formula is C19H33NO2, and its molecular weight is approximately 307.5 g/mol.
Fingolimod blocks the migration and homing of lymphocytes to the central nervous system through its interaction with sphingosine 1-phosphate receptors and exhibits potential anti-inflammatory properties.
Impurities in Fingolimod can be unintended byproducts that are present with the active ingredient. They form during the synthetic1 process, storage, or due to interactions with other substances. Rigorous analysis and control measures help ensure product quality and safety. Analytical techniques such as chromatography and spectroscopy help identify, characterize, and quantify these impurities. Stringent manufacturing practices help to minimize impurity formation and maintain the purity of Fingolimod.
Daicel Pharma, in adherence to cGMP standards, operates an analytical facility where we prepare Fingolimod impurity standards like 2-Phenethyl Fingolimod Impurity, 3-Phenethyl Fingolimod Impurity, Fingolimod Impurity-1, Fingolimod impurity-3, and Fingolimod Phosphate. We offer a comprehensive Certificate of Analysis (CoA) for these impurities, providing a detailed characterization report. The CoA includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Fingolimod impurities, degradation products, and labeled compounds to assess the effectiveness of generic Fingolimod. For bio-analytical research, including BA/BE studies, we offer Fingolimod-D4, a deuterated-labeled standard of Fingolimod. Each delivery has a comprehensive characterization report.
The control measures used during Fingolimod manufacturing are strict monitoring of process parameters, optimization of reaction conditions, adequate purification techniques, implementation of appropriate storage, and packaging conditions to minimize impurity formation.
Yes, regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established regulatory guidelines specifying the acceptable limits of impurities in Fingolimod to ensure product quality and patient safety.
Methanol or acetonitrile are the commonly used solvents when analyzing many impurities in Fingolimod.
The recommended temperature to store Fingolimod impurities is at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.