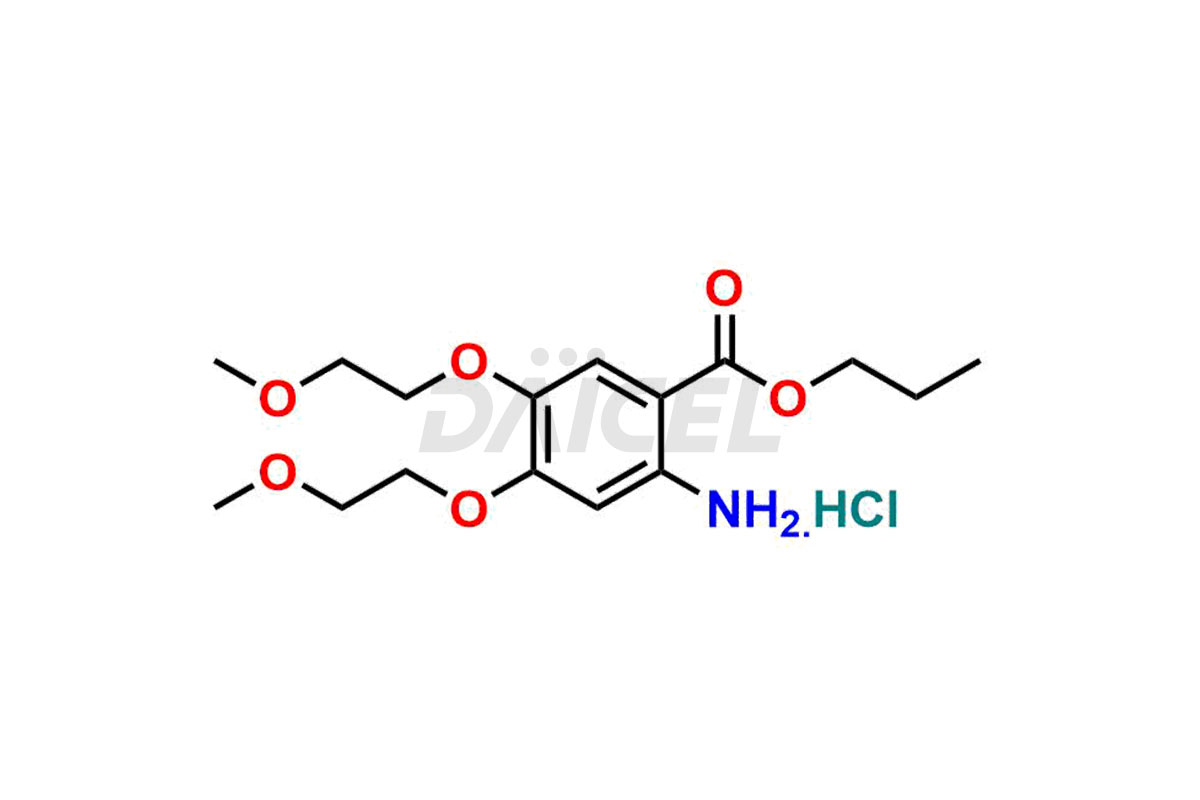
Erlotinib
LOAD MORE
You're viewed all 19 products
References
FAQ's
References
- Schnur, Rodney C.; Arnold, Lee D., Quinazoline Derivatives, Pfizer Inc., United States, EP817775B1, June 9, 1995
- Pujeri, S. S.; Khader, A. M. A.; Seetharamappa, J., Validated Stability-Indicating Chromatographic Method for the Assay of Erlotinib Active Pharmaceutical Ingredient, Analytical Letters, Volume: 42, Issue: 12, Pages: 1855-1867, 2009
Frequently Asked Questions
How can process-related impurities be detected in Erlotinib?
The rapid, reversed-phase high-performance liquid chromatographic (HPLC) method helps detect and analyze Erlotinib and its impurities.
How to prevent impurities from forming in Erlotinib during storage?
Erlotinib is stored in a cool, dry place and protected from light and moisture to prevent the formation of impurities.
Which solvent helps in the analysis of Erlotinib impurities?
Methanol is one of the solvents used in analyzing most of the Erlotinib impurities.
What are the temperature conditions required to store Erlotinib impurities?
Erlotinib impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.