Efavirenz Impurities
General Information
Efavirenz Impurities and Efavirenz
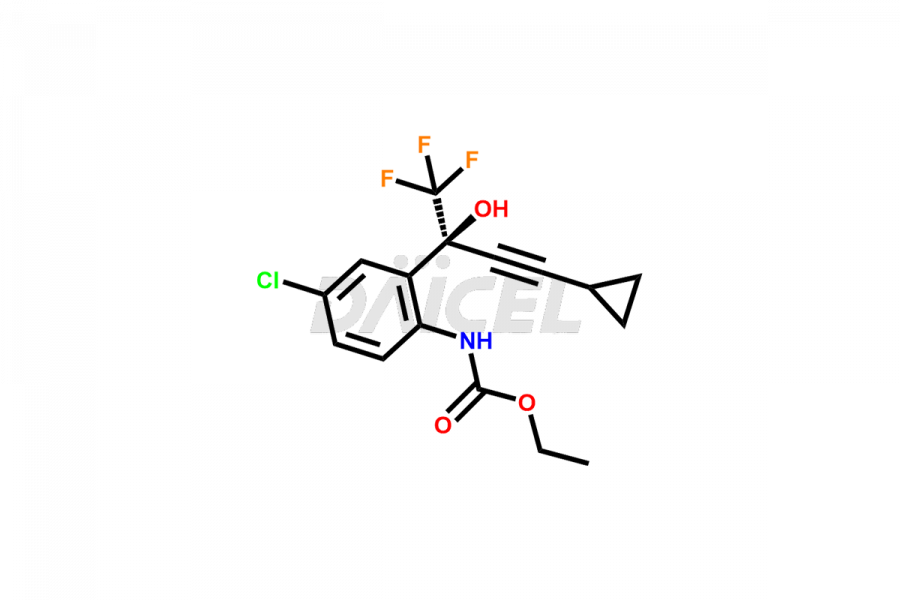
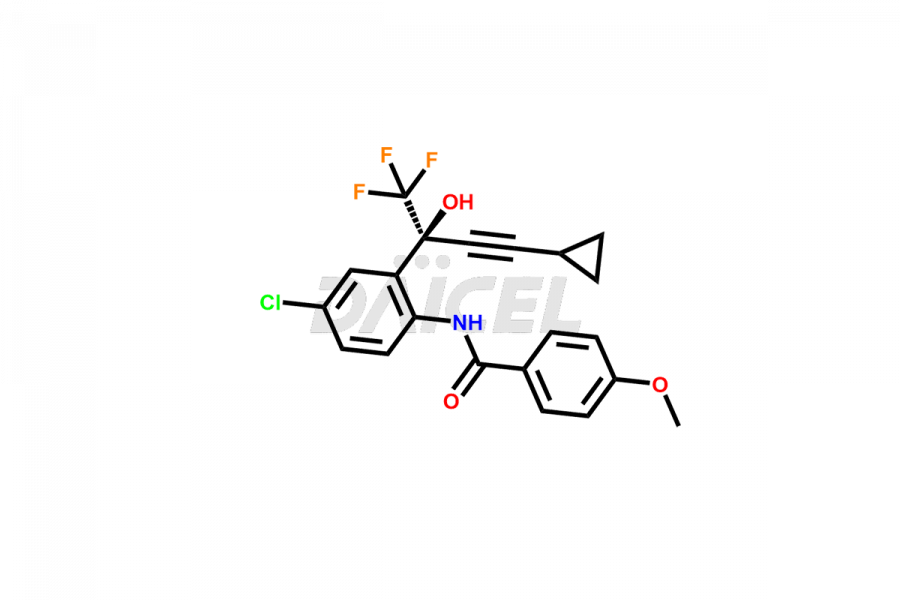
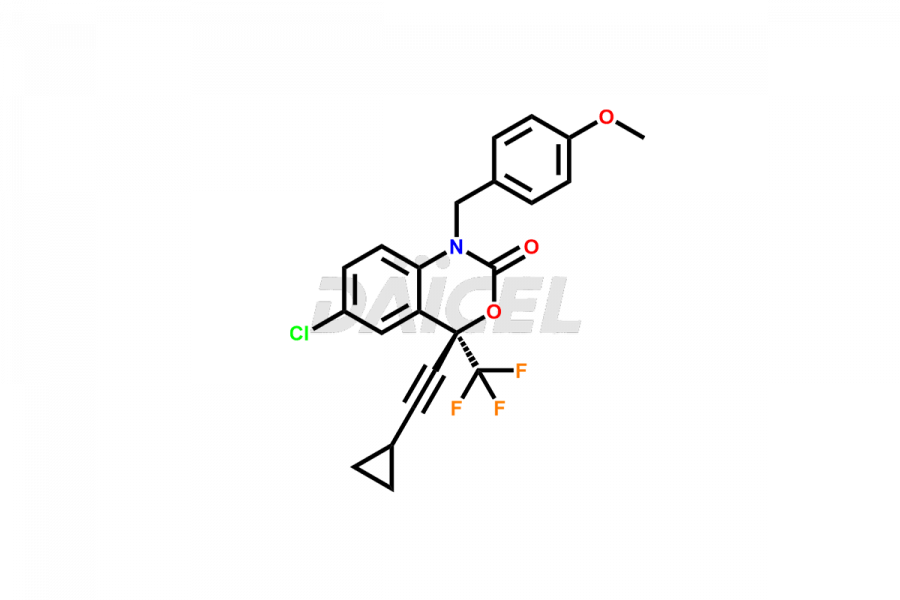
Daicel Pharma offers high-quality Efavirenz impurities, Efavirenz Amino Alcohol, Efavirenz Amino alcohol ethyl carbamate, Efavirenz Amino Alcohol Methyl Carbamate, Efavirenz Amino alcohol Bis(ethoxycarbonyl), Efavirenz Benzoyl Amino Impurity, Efavirenz Quinoline analog, and N-Benzyl Efavirenz. They are vital for evaluating the quality, stability, and biological safety of Efavirenz. Furthermore, Daicel Pharma specializes in the custom synthesis of Efavirenz impurities and ensures their worldwide delivery.
Efavirenz [CAS: 154598-52-4] is USFDA-approved for treating and preventing human immunodeficiency virus (HIV) and HIV-1 infection. It is a non-nucleoside reverse transcriptase inhibitor (NNRTI), which is HIV-1 specific. Efavirenz, used in combination with other HIV medicines, is a part of antiretroviral therapy.
Efavirenz: Use and Commercial Availability
Efavirenz is a drug for treating HIV-1 infection in patients. Efavirenz is a part of the antiretroviral therapy (ART) regimens. The treatment involves its combination with other HIV medicines like Tenofovir or Emtricitabine. Treatment with Efavirenz can improve the quality of life of patients affected with HIV. Efavirenz is available under the name Sustiva.
Efavirenz Structure and Mechanism of Action
The chemical name of Efavirenz is (4S)-6-Chloro-4-(2-cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one. The chemical formula for Efavirenz is C14H9ClF3NO2 and its molecular weight is approximately 315.68g/mol.
As a non-nucleoside reverse transcriptase inhibitor (NNRTI), Efavirenz binds to the non-catalytic site of the HIV reverse transcription enzyme. Efavirenz activity inhibits HIV-1 RT. It results in DNA chain termination and prevents HIV replication.
Efavirenz Impurities and Synthesis
During the synthesis of Efavirenz1, impurities may form that may affect the safety and efficacy of the drug. These impurities form during the synthesis, storage, or degradation of Efavirenz. As a result, impurities must be controlled and monitored throughout the drug’s lifecycle.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Efavirenz impurities, which includes Efavirenz Amino Alcohol, Efavirenz Amino alcohol ethyl carbamate, Efavirenz Amino Alcohol Methyl Carbamate, Efavirenz Aminoalcohol Bis(ethoxycarbonyl), Efavirenz Benzoyl Amino Impurity, Efavirenz Quinoline analog, and N-Benzyl Efavirenz. The CoA is from a cGMP-compliant analytical facility and encompasses complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional data like 13C-DEPT and CHN on request. Daicel Pharma can prepare any unidentified Efavirenz impurity or degradation product. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Efavirenz for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- Young, Steven D.; Tran, Lekhanh O.; Britcher, Susan F.; Lumma, William C., Jr.; Payne, Linda S., Benzoxazinones as inhibitors of HIV reverse transcriptase, EP582455, Feb 9, 1994, Merck and Co., Inc., United States
- Villani, Paola; Pregnolato, Massimo; Banfo, Simona; Rettani, Mauro; Burroni, Daniela; Seminari, Elena; Maserati, Renato; Regazzi, Mario B., Therapeutic Drug Monitoring, Volume: 21, Issue: 3, Pages: 346-350, 1999
Frequently Asked Questions
What are the effects of impurities in Efavirenz?
Impurities may lower the drug shelf life, change the physical and chemical properties, decrease the therapeutic effects, and more.
How can the formation of impurities be minimized during the synthesis?
The formation of impurities can be minimized by optimizing the synthetic process, using high-quality raw materials, and maintaining strict quality control measures.
How do you detect impurities of Efavirenz?
Gradient reversed phase high-performance liquid chromatography (RP-HPLC) helps detect Efavirenz impurities.
How do you identify the genotoxic Impurity in the Efavirenz drug product?
LC-MS/MS helps identify the genotoxic Impurity in the Efavirenz drug product.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.