Digoxin
General Information
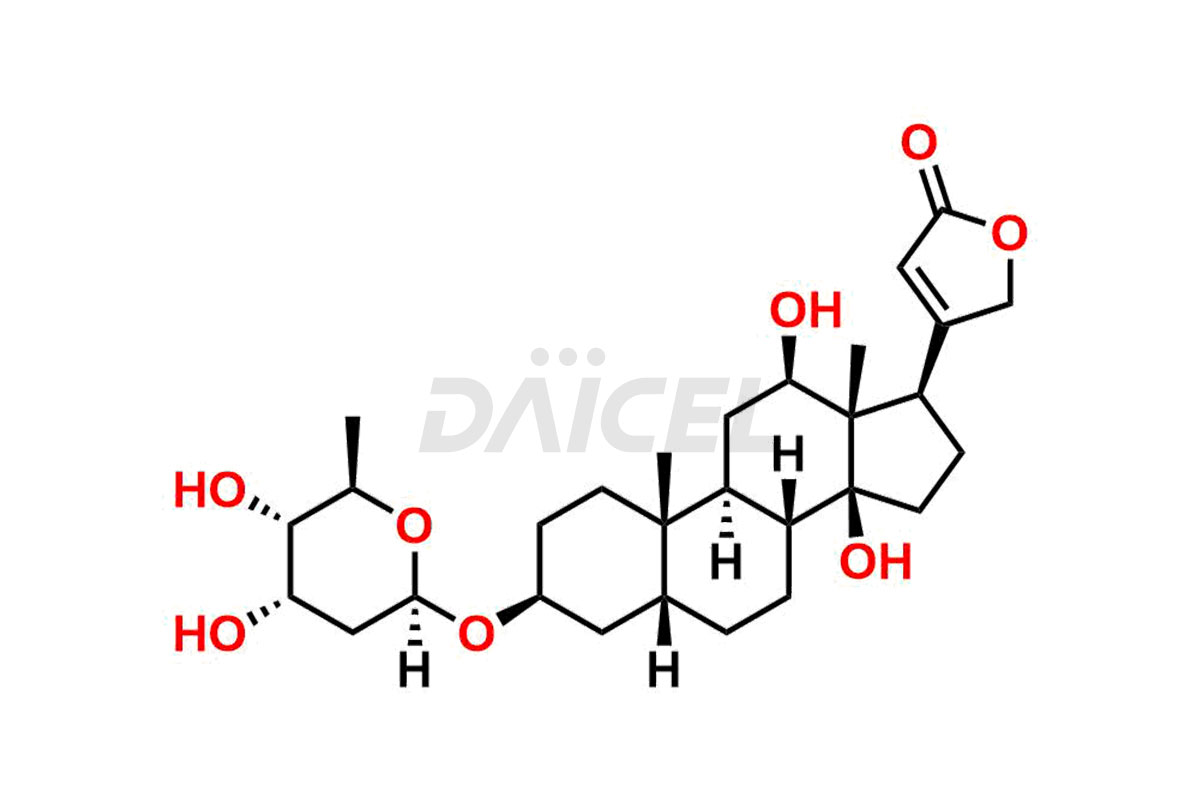
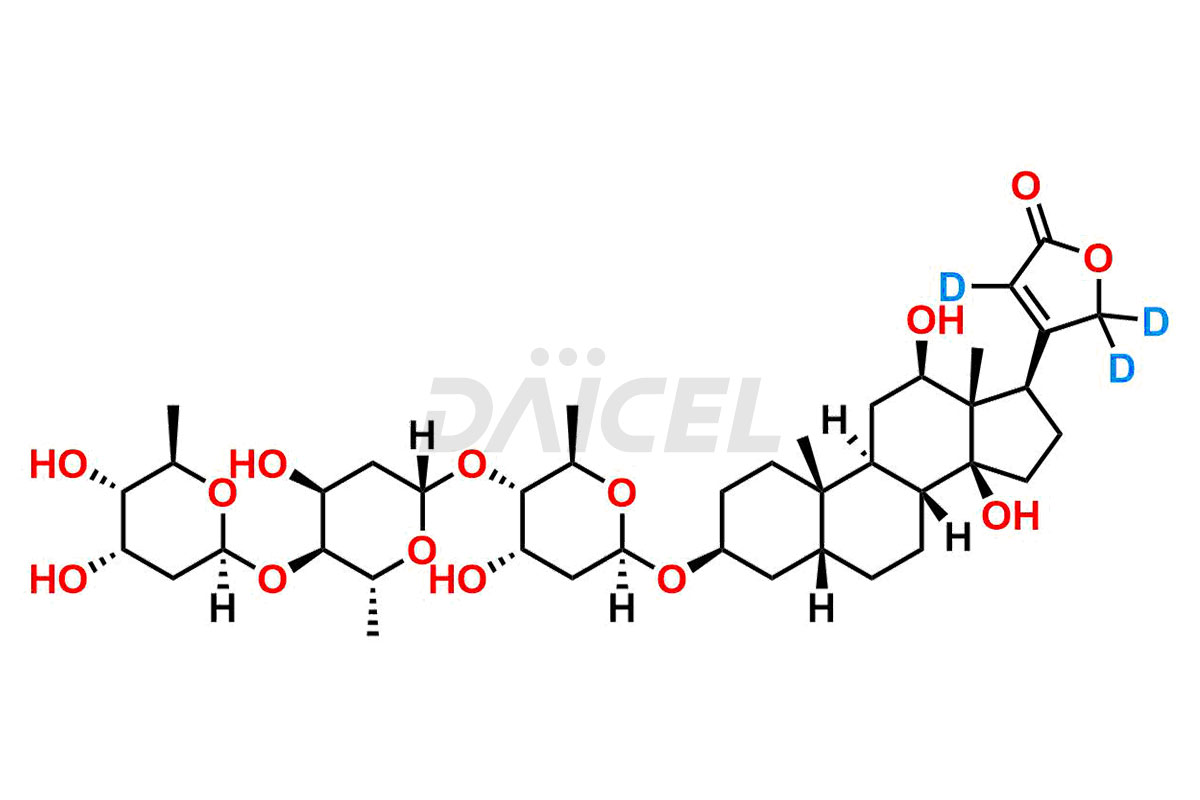
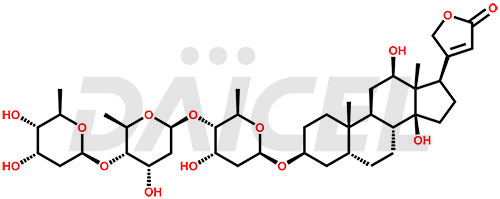
Digoxin Impurities and Digoxin
Daicel synthesizes high-quality impurities for Digoxin, an active pharmaceutical ingredient. These impurities, including Digoxin impurity-F and Digoxin Impurity-D, play a vital role in assessing Digoxin’s purity, reliability, and safety. Daicel Pharma also offers a customized synthesis of Digoxin impurities to cater to client requirements, with worldwide delivery options available.
Digoxin [CAS: 20830-75-5], a cardiac glycoside extracted from the foxglove plant, digitalis, treats heart conditions such as atrial fibrillation, atrial flutter, and congestive heart failure. It possesses positive inotropic and negative chronotropic activity. Digoxin controls ventricular rate in atrial fibrillation and manages congestive heart failure associated with atrial fibrillation.
Digoxin: Use and Commercial Availability
Digoxin is a cardiotonic glycoside derived from the Digitalis lanata plant, commonly known as foxgloves. It belongs to the digitalis class and treats heart conditions such as atrial flutter, atrial fibrillation, and heart failure. It is particularly effective in mild to moderate heart failure and in improving myocardial contraction. Digoxin is for patients with systolic heart failure (HFrEF) with an ejection fraction below 40% but does not reduce mortality. It is for rate control in atrial fibrillation or atrial flutter. Digoxin controls supraventricular tachycardias that are unresponsive to conventional therapies. Digoxin is available under brand names, including Digoxin Pediatric, Lanoxicaps, Lanoxin, and Lanoxin Pediatric.
Digoxin Structure and Mechanism of Action 
The chemical name of Digoxin is (3β,5β,12β)-3-[(O-2,6-Dideoxy-β-D-ribo-hexopyranosyl-(1→4)-O-2,6-dideoxy-β-D-ribo-hexopyranosyl-(1→4)-2,6-dideoxy-β-D-ribo-hexopyranosyl)oxy]-12,14-dihydroxycard-20(22)-enolide. Its chemical formula is C41H64O14, and its molecular weight is approximately 780.9 g/mol.
Digoxin inhibits an enzyme, sodium-potassium ATPase regulating the quantity of sodium and potassium inside cells.
Digoxin Impurities and Synthesis
Digoxin impurities form during the synthesis1,2, storage, or degradation of the drug. They may arise from impure starting materials, reaction by-products, or environmental factors. The analysis and control of Digoxin impurities are essential to ensure the drug’s quality, efficacy, and safety. Impurities can impact the drug’s potency, stability, and potential adverse effects. Therefore, thorough analysis and control of Digoxin impurities are necessary to meet regulatory requirements and ensure patient well-being. It involves identifying and quantifying measures to minimize impurity formation during manufacturing, storage, and distribution.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Digoxin impurity standards, including Digoxin Impurity-F and Digoxin Impurity-D. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis3. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Digoxin impurities or degradation products, and labeled compounds, to evaluate the efficacy of generic Digoxin. Also, Digoxin-D3, a deuterium-labeled Digoxin standard, is available for bio-analytical research, including BA/BE studies. Every delivery accompanies a complete characterization report.
References
FAQ's
References
- Nozaki, Yoshio; Mayama, Mikao; Akaki, Koji; Satoh, Daisuke, Microbiological 12β-hydroxylation of digitoxin, a steroidal glycoside, Agricultural and Biological Chemistry, Volume: 29, Issue: 8, Pages: 783, 1965
- Smith, Sydney, Digoxin, a new digitalis glucoside, Journal of the Chemical Society, Pages: 508-10, 1930
- Watson, Eric; Kalman, Sumner M., Assay of digoxin in plasma by gas chromatography, Journal of Chromatography, Volume: 56, Issue: 2, Pages: 209-18, 1971
Frequently Asked Questions
How can Digoxin impurities impact the drug's stability?
Digoxin impurities can contribute to the degradation of the drug over time, reducing its shelf life and stability. Controlling them is crucial for maintaining the drug's long-term stability.
How are Digoxin impurities monitored during manufacturing?
During manufacturing, Digoxin impurities are monitored through regular testing and analysis at different stages of the production process. It ensures that impurity levels are within acceptable limits.
Which solvent helps in the analysis of Digoxin impurities?
Methanol is a solvent used in analyzing many impurities in Digoxin.
What are the temperature conditions required to store Digoxin impurities?
Digoxin impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.