Cobicistat
General Information
Cobicistat Impurities and Cobicistat
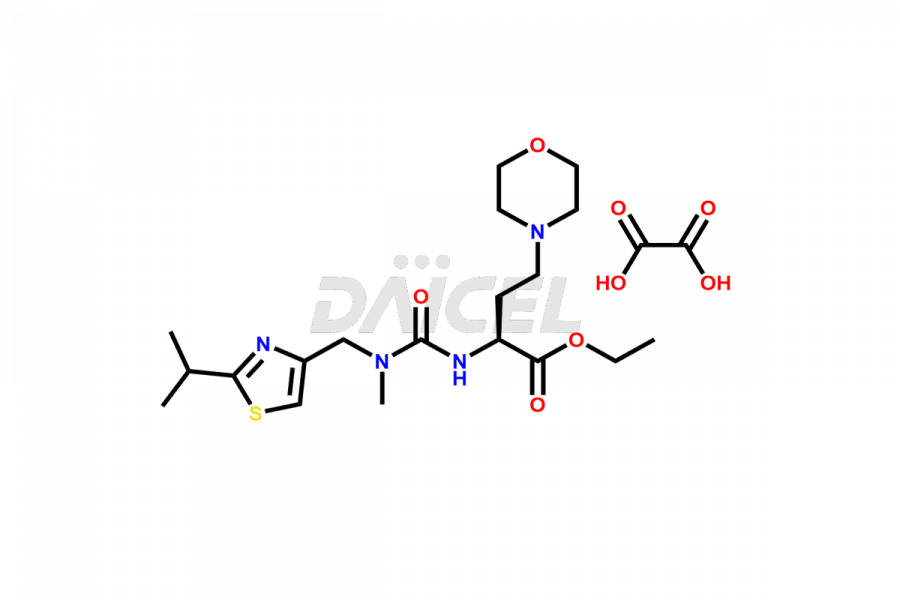
Daicel Pharma offers the best-quality Cobicistat impurities, such as 5-Thiazolylmethyl N-[(1R,4R)-4-Amino-5-phenyl-1-(phenylmethyl)pentyl] Carbamate (TFA Salt) and Ethyl(S)-2-(3-((2-isopropylthiazol-4-yl)methyl)-3-methylureido)-4-morpholinobutanoate oxalate. It is vital to evaluate Cobicistat quality, stability, and biological safety. Further, Daicel Pharma is an expert in custom synthesizing Cobicistat impurities and ensuring their delivery globally.
Cobicistat [CAS: 1004316-88-4] is a monocarboxylic acid amide and pharmacokinetic enhancer for boosting other HIV medicine activity. It was developed by Gilead Sciences Inc. Cobicistat treats HIV-1 infections in combination with Atazanavir or Darunavir. It promotes the anti-HIV effect of Atazanavir or Darunavir by blocking human cytochrome P-450 3A (CYP3A) enzymes. It is a mechanism-based inhibitor.
Cobicistat: Use and Commercial Availability
Cobicistat is a non-antiretroviral medicine. It is a medication booster of other antiretrovirals and is a part of HIV treatment therapy. It increases systemic exposure to antiretroviral and does not increase dosage, thus providing better treatment options. Cobicistat is available as Tybost for treating human immunodeficiency virus (HIV) infection.
Cobicistat Structure and Mechanism of Action
The chemical name of Cobicistat is (3R,6R,9S)- 12-methyl-13-[2-(1-methylethyl)-4-thiazolyl]-9-[2-(4-morpholinyl)ethyl]-8,11-dioxo-3,6-bis(phenylmethyl)- 2,7,10,12-Tetraazatridecanoic acid 5-thiazolylmethyl ester. The chemical formula for Cobicistat is C40H53N7O5S2, and its molecular weight is approximately 776.03 g/mol.
Cobicistat blocks the metabolism of cytochrome P450 3A (CYP3A). It increases the systematic exposure of atazanavir and darunavir, CYP3A substrates, thus increasing anti-viral activity at a lower dosage.
Cobicistat Impurities and Synthesis
During Cobicistat synthesis1, impurities form that may affect the safety and efficacy of the drug. They form during the synthetic process, purification, or storage of Cobicistat. And so, Cobicistat impurities must be controlled and monitored throughout the drug development process.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Cobicistat impurities, which includes 5-Thiazolylmethyl N-[(1R,4R)-4-Amino-5-phenyl-1-(phenylmethyl)pentyl] Carbamate (TFA Salt) and Ethyl(S)-2-(3-((2-isopropylthiazol-4-yl)methyl)-3-methylureido)-4-morpholinobutanoate oxalate. The CoA is from a cGMP-compliant analytical facility. It provides complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional analytical data on request. Daicel Pharma can prepare any unidentified Cobicistat impurity or degradation product. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Cobicistat for bioanalytical research and BA/BE studies. The clients of Daicel Pharma can expect a complete characterization report on delivery.
References
FAQ's
References
- Desai, Manoj C.; Hong, Allen Yu; Liu, Hongtao; Xu, Lianhong; Vivian, Randall W., Modulators of pharmacokinetic properties of therapeutics, WO2008010921A2, Jan 24, 2008, Gilead Sciences, Inc., United States (https://patents.google.com/patent/WO2008010921A2/en)
- Alekya, Padala; Varalakshmi, Kurla Venkata; Pawar, A. K. M., Quality by design (QbD) based development of a stability indicating RP-HPLC method for estimation of cobicistat in bulk, International Journal of Pharmaceutical Sciences and Research, Volume: 9, Issue: 6, Pages: 2589-2594, 2018 DOI: (10.13040/ijpsr.0975-8232.9(6).2589-94)
Frequently Asked Questions
2. How do Cobicistat impurities form in the drug substance?
Unreacted raw materials, intermediates, by-products, residual solvents, reagents, and catalysts form Cobicistat impurities in the drug.
3. Why do we need to control Cobicistat impurities in the drug substance?
The presence of Cobicistat impurities in the drug substance will affect the drug’s safety and quality. Hence, it is essential to remove them from the drug substance.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.