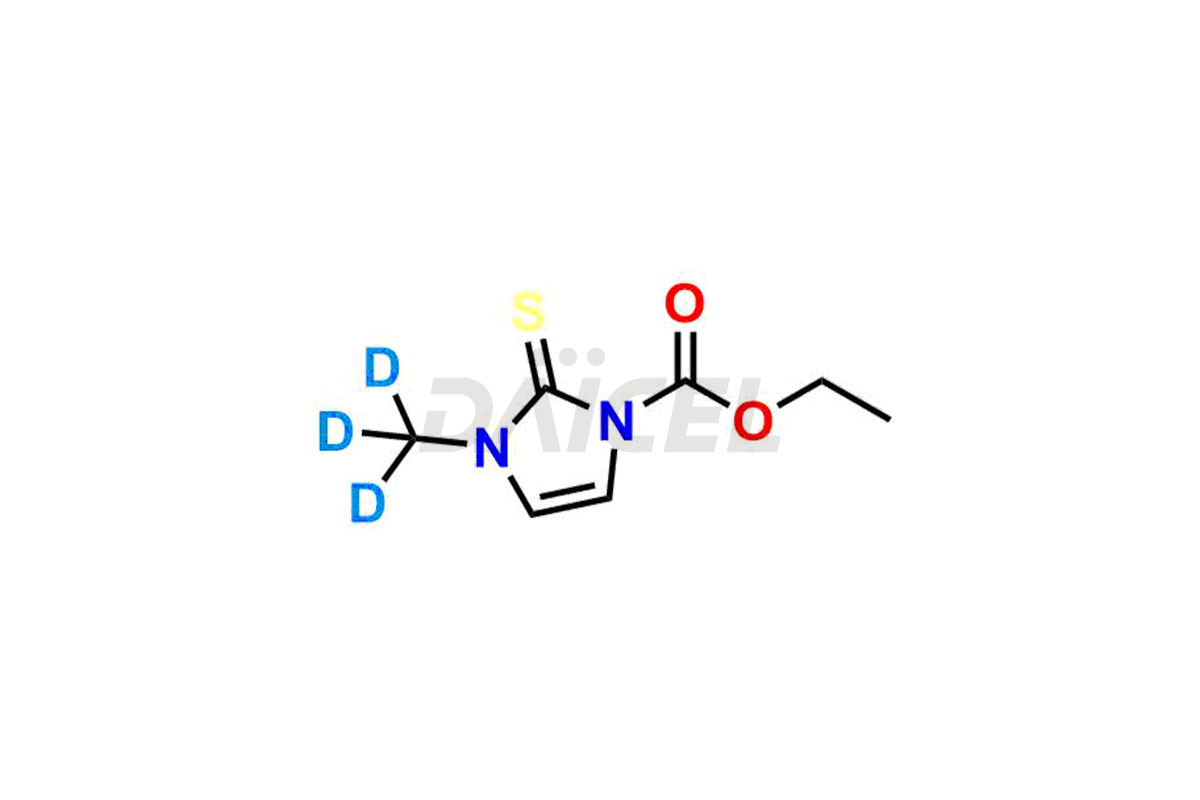
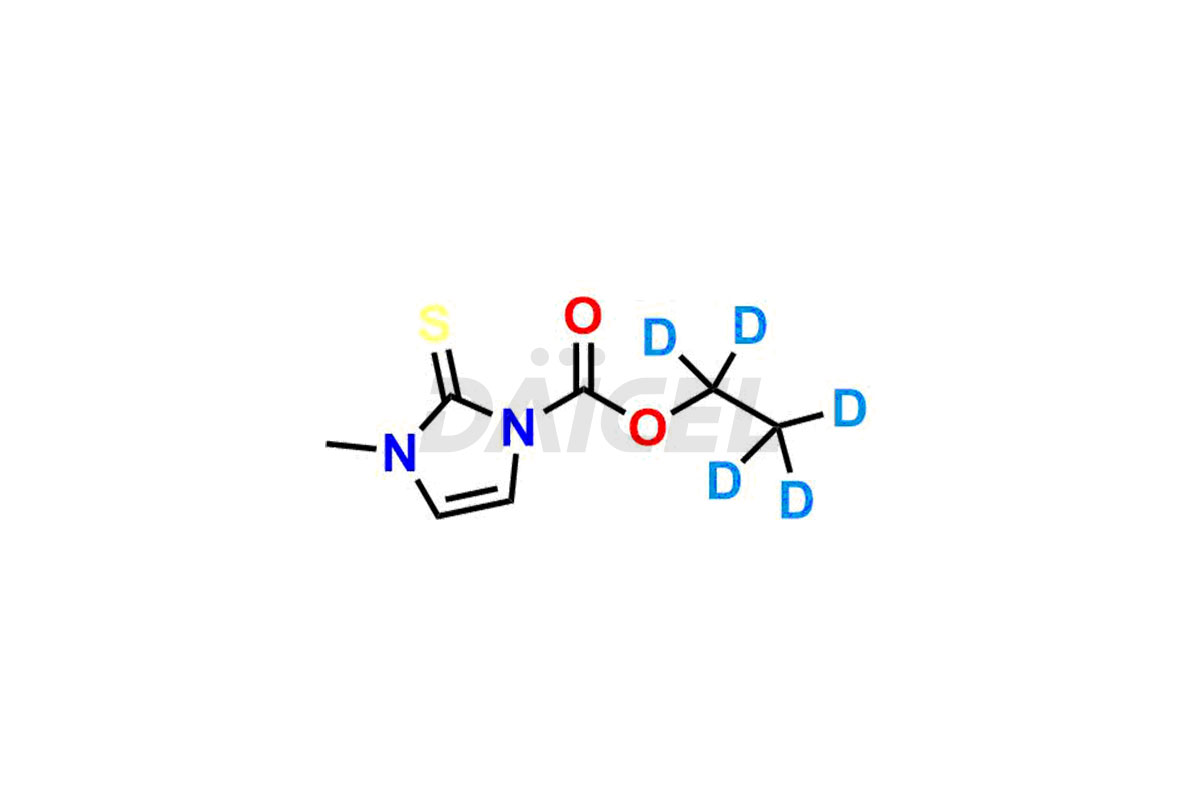
Carbimazole
General Information
Carbimazole Impurities and Carbimazole
Daicel Pharma offers superior-quality Carbimazole impurities and labeled standards. It is vital for evaluating Carbimazole quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Carbimazole impurities and ensures worldwide delivery.
Carbimazole [CAS: 22232-54-8] is an imidazole derivative, which is a prodrug for methimazole. It treats hyperthyroidism. It is a thionamide drug that normalizes thyroid hormone. It inhibits the iodination of tyrosine in thyroglobulin, a glycoprotein produced by the thyroid gland.
Carbimazole: Use and Commercial Availability
Carbimazole treats hyperthyroidism by minimizing excess thyroid hormone production. It also treats thyrotoxicosis, wherein high levels of thyroid hormones, triiodothyronine (T3), and thyroxine (T4) circulate in the body. Carbimazole is available as oral tablets of different strengths. Generic manufacturers sell Carbimazole under various brands, such as Atirozidina, Thyrostat, Basolest, Mertiran, etc.
Carbimazole Structure and Mechanism of Action
The chemical name of Carbimazole is Ethyl 3-methyl-2-thioxo-2,3-dihydro-1H-imidazole-1-carboxylate. The chemical formula for Carbimazole is C7H10N2O2S, and its molecular weight is approximately 186.23 g/mol.
As an antithyroid agent, Carbimazole decreases the formation of di-iodotyrosine and thyroxine. It reduces the uptake of inorganic iodine by the thyroid gland.
Carbimazole Impurities and Synthesis
When synthesizing Carbimazole 1, the formation of impurities may affect drug safety and efficacy. They form during the synthetic process, storage, or purification of Carbimazole. Carbimazole impurities need control and monitoring to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Carbimazole impurities and labeled standards. A CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data2 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Carbimazole impurity or degradation product. In addition, Daicel Pharma offers highly purified, stable deuterium-labeled standards, Carbimazole Labelled Standard, and Labelled Standard. Daicel Pharma provides a complete characterization report accompanying the delivery.
References
FAQ's
References
- Rimington, Claude; Lawson, Alexander; Searle, Charles E.; Morley, Harold V., Certain substituted thioglyoxalones, US2815349A, Dec 3, 1957, National Research Development Corp. (https://www.lens.org/lens/search/patent/list?q=US2815349)
- El-Bardicy, M. G.; El-Saharty, Y. S.; Tawakkol, M. S., Determination of carbimazole and methimazole by first and third derivative spectrophotometry, Spectroscopy Letters, Volume: 24, Issue: 9, Pages: 1079-95, 1991 DOI: (10.1080/00387019108018174)
Frequently Asked Questions
2. Which analytical methods separate and qualify the main Carbimazole impurity and degradation product from the drug product?
Stability-indicating HPLC and HPTLC methods separate and qualify the main Carbimazole impurity and degradation product from the drug product.
3. How do the degradation products of Carbimazole form?
The degradation products of Carbimazole are formed under thermal, photolytic, oxidative, and hydrolytic conditions.
4. Which analytical methods confirm the degradation products of Carbimazole?
MS and IR spectroscopy are the methods to characterize and confirm the degradation products of Carbimazole.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.