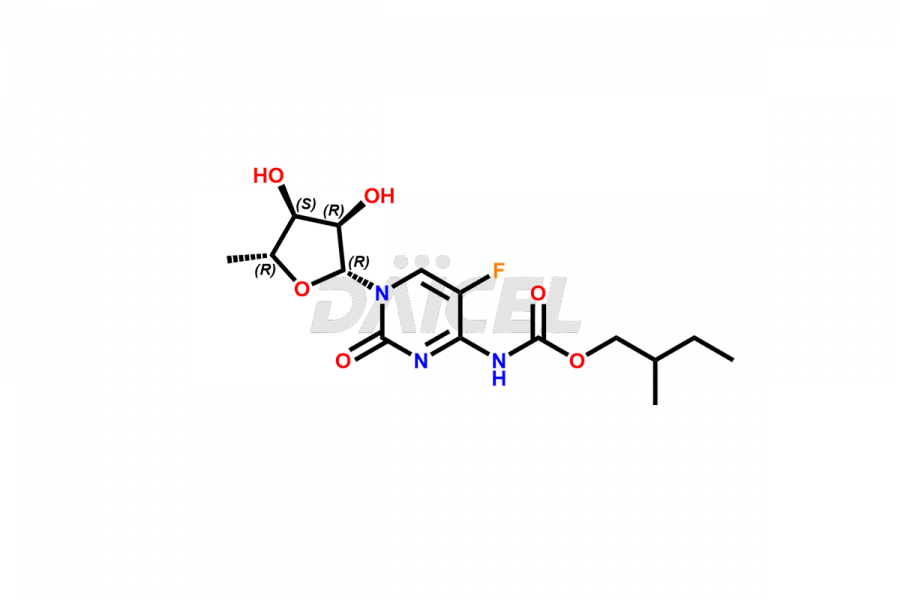
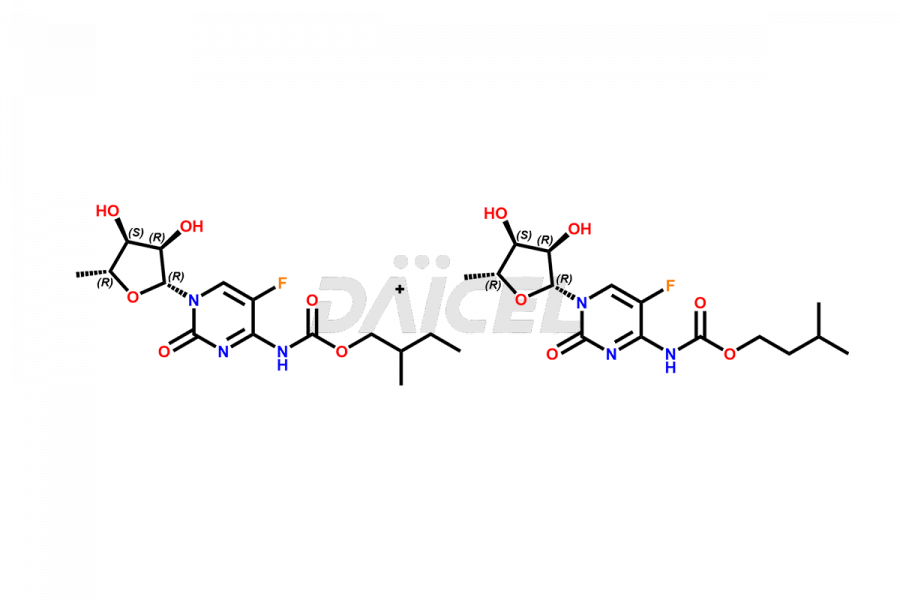
Capecitabine Impurities
General Information
Capecitabine Impurities and Capecitabine
Daicel Pharma offers high-quality Capecitabine impurities, Capecitabine EP impurity D, Capecitabine EP impurity E, and Capecitabine Isomers Mixture. They are vital for evaluating the quality, stability, and biological safety of Capecitabine. Furthermore, Daicel Pharma specializes in the custom synthesis of Capecitabine impurities and ensures their worldwide delivery.
Capecitabine [CAS: 154361-50-9] is an antineoplastic agent for cancer treatment. It belongs to the category of carbamate esters. It is a prodrug of 5’-deoxy-5-fluorouridine (5’-DFUR) and converted to 5-fluorouracil.
Capecitabine: Use and Commercial Availability
Capecitabine is a fluoropyrimidine carbamate, a type of antineoplastic agent. It treats patients with metastatic breast cancer resistant to both paclitaxel and an anthracycline-containing chemotherapy regimen. Capecitabine, marketed under the brand name Xeloda, is utilized as an antimetabolite in the treatment of colon cancer, breast cancer, and gastric cancer.
Capecitabine Structure and Mechanism of Action
The chemical name of Capecitabine is 5′-Deoxy-5-fluoro-N-[(pentyloxy)carbonyl]cytidine. The chemical formula for Capecitabine is C15H22FN3O6, and its molecular weight is approximately 359.35g/mol.
Capecitabine activation is by tumor cells selectively to its cytotoxic moiety, 5-fluorouracil (5-FU). Further, 5-FU metabolizes to two active metabolites, 5-fluoro-2-deoxyuridine monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP).
Capecitabine Impurities and Synthesis
While synthesizing Capecitabine1, stringent protocols can uphold its purity and effectiveness. However, impurities may arise during the process, posing challenges to drug quality. Advanced purification methods such as chromatography and crystallization can mitigate impurities. Continuous vigilance and adherence to rigorous quality control measures are imperative to ensure the integrity of Capecitabine throughout its synthesis.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Capecitabine impurities, which includes Capecitabine EP impurity D, Capecitabine EP impurity E, and Capecitabine Isomers Mixture. The CoA is from a cGMP-compliant analytical facility and encompasses complete characterization data such as 1H NMR, 13C NMR2, IR, MASS, and HPLC purity. On request, we give additional data like 13C-DEPT and CHN. Daicel Pharma also prepares any unidentified Capecitabine impurity or degradation product. Furthermore, Daicel Pharma offers highly purified isotope-labeled standards of Capecitabine for bioanalytical research and BA/BE studies. We provide a complete characterization report upon delivery.
References
FAQ's
References
- Arasaki, Motohiro Nippon Roche; Ishitsuka, Hideo; Kuruma, Isami; Miwa, Masanori; Murasaki, Chikako; Shimma, Nobuo; Umeda, Isao Imperial Higashihak, N-Oxycarbonyl substituted 5'-deoxy-5-fluorocytidines, EP602454, June 22, 1994, F. Hoffmann-La Roche & Co. AG, Switzerland
- Xu, Yan; Grem, Jean L., Liquid chromatography-mass spectrometry method for the analysis of the anticancer agent Capecitabine and its nucleoside metabolites in human plasma, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 783, Issue: 1, Pages: 273-285, 2003 DOI: (10.1016/s1570-0232(02)00674-8)
Frequently Asked Questions
How can Capecitabine impurities be minimized during the synthesis of the drug?
Capecitabine impurities are minimized through careful selection of raw materials, optimization of synthetic routes, purification techniques, and stringent quality control measures.
What is the role of impurity profiling in the synthesis of Capecitabine?
Impurity profiling helps understand the nature and quantity of impurities in pharmaceuticals, which is crucial for the safety and efficacy of the drug.
Can Capecitabine impurities impact the stability of the drug over time?
Impurities can contribute to the degradation of Capecitabine over time, leading to reduced stability and shelf-life of the drug product.
How can impurity profiles vary between different batches of Capecitabine?
Variations in impurity profiles between batches of Capecitabine can arise from differences in synthesis processes, raw materials, or storage conditions.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.