Branebrutinib
General Information
Branebrutinib Impurities and Branebrutinib
Daicel Pharma offers high-quality Branebrutinib impurities, such as Branebrutinib Enantiomer. It is vital for evaluating the quality, stability, and biological safety of Branebrutinib. Furthermore, Daicel Pharma specializes in the custom synthesis of Branebrutinib impurities and ensures their worldwide delivery.
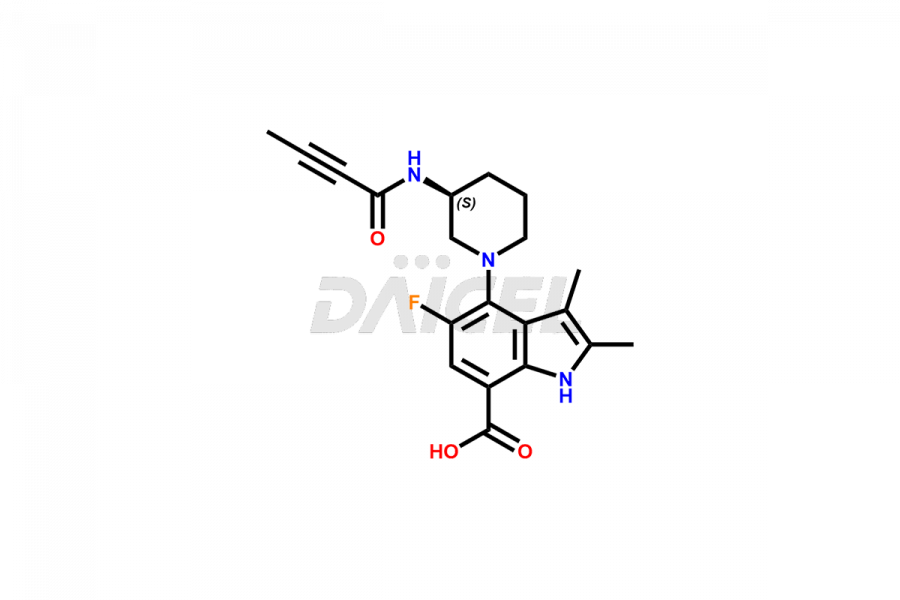
Branebrutinib (BMS‐986195) [CAS: 1912445-55-6] is an oral, reversible inhibitor of Bruton’s tyrosine kinase. It exhibits potential in treating immune-mediated and inflammatory conditions and B-cell malignancies. It is a small molecule, a covalent inhibitor of BTK.
Branebrutinib: Use and Commercial Availability
Branebrutinib can reverse multidrug resistance (MDR) in cancer cells mediated by P-glycoprotein (P-gp). Branebrutinib is under clinical investigation.
Branebrutinib Structure and Mechanism of Action


The chemical name of Branebrutinib is 5-Fluoro-2,3-dimethyl-4-[(3S)-3-[(1-oxo-2-butyn-1-yl)amino]-1-piperidinyl]-1H-indole-7-carboxamide. The chemical formula for Branebrutinib is C20H23FN4O2, and its molecular weight is approximately 370.4g/mol.
The mechanism of action of Branebrutinib is not known.
Branebrutinib Impurities and Synthesis
During the synthesis1 of Branebrutinib, meticulous procedures can ensure its purity and potency. Despite these efforts, impurities can arise, potentially affecting the drug’s quality. Advanced purification techniques such as chromatography and crystallization can minimize impurities. Continuous monitoring and adherence to strict quality control protocols are essential to maintain the integrity of Branebrutinib throughout its synthetic process.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Branebrutinib impurities such as Branebrutinib Enantiomer. The CoA is from a cGMP-compliant analytical facility and encompasses complete characterization data such as 1H NMR, 13C NMR, IR, MASS2, and HPLC purity. On request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can prepare any unidentified Branebrutinib impurity or degradation product. Furthermore, Daicel Pharma offers highly purified isotope-labeled standards of Branebrutinib for bioanalytical research and BA/BE studies. We provide a complete characterization report upon delivery.
References
FAQ's
References
- Ahmad, Saleem; Tino, Joseph A.; Macor, John E.; Tebben, Andrew J.; Gong, Hua; Liu, Qingjie; Batt, Douglas G.; Ngu, Khehyong; Watterson, Scott Hunter; Guo, Weiwei; et al, Indole Carboxamides Compounds Useful As Kinase Inhibitors, WO2016065226A1, April 28, 2016, Bristol-Myers Squibb Company, United States
- Zheng, Naiyu ; Catlett, Ian M.; Taylor, Kristin; Gu, Huidong ; Pattoli, Mark A.; Neely, Robert J.; Li, Wenying; Allentoff, Alban; Yuan, Xiling; Ciccimaro, Eugene; et al, Determination of Real Time in Vivo Drug Receptor Occupancy for a Covalent Binding Drug as a Clinical Pharmacodynamic Biomarker by Immunocapture-LC-MS/MS, Analytical Chemistry (Washington, DC, United States), Volume: 91, Issue: 13, Pages: 8443-8452, 2019, DOI: (10.1021/acs.analchem.9b01462)
Frequently Asked Questions
How frequently should impurity levels in Branebrutinib be monitored during synthesis?
Impurity levels in Branebrutinib should be monitored regularly throughout the synthetic process to ensure compliance with regulatory standards and maintain product quality.
What are the uses of Branebrutinib impurities?
Branebrutinib impurities help in pharmaceutical research, product development, ANDA and DMF filing, quality control (QC), method validation, and stability studies.
What are the safety measures during the synthesis of Branebrutinib impurities?
Safety measures during the synthesis of Branebrutinib impurities include personal protective equipment (PPE), proper ventilation, and adherence to standard operating procedures (SOPs).
What are the safety measures during the storage of Branebrutinib impurities?
Safety measures during the storage of Branebrutinib impurities include proper labeling, secure storage conditions, and regular monitoring for any signs of degradation.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.