Blonanserin Impurities
General Information
Blonanserin Impurities and Blonanserin
Daicel Pharma offers high-quality Blonanserin impurities, Blonanserin C TFA, and genotoxic N-Nitroso desethyl Blonanserin impurity. They are vital for evaluating Blonanserin’s quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Blonanserin impurities and ensures their worldwide delivery.
Blonanserin [CAS: 132810-10-7] (BNN) is an antipsychotic medication endorsed for managing schizophrenia in Japan and Korea. It targets dopamine D2 and serotonin 5-HT2A receptors in patients.
Blonanserin: Use and Commercial Availability
Blonanserin, an atypical antipsychotic, received approval in Japan in January 2008. Notably, it boasts enhanced tolerability by avoiding adverse effects like extrapyramidal symptoms, excessive sedation, or hypotension. Being a second-generation (atypical) antipsychotic, it treats the negative symptoms of schizophrenia compared to first-generation (typical) antipsychotics. It is marketed under the brand name Lonasen.
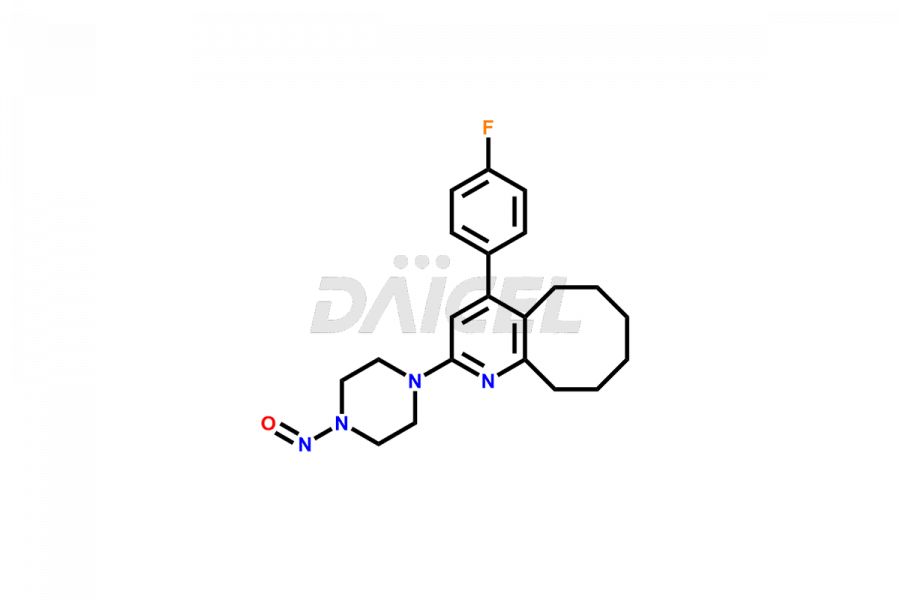
Blonanserin Structure and Mechanism of Action
The chemical name of Blonanserin is 2-(4-Ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridine. The chemical formula for Blonanserin is C23H30FN3, and its molecular weight is approximately 367.5g/mol.
Blonanserin binds and inhibits dopamine receptors D2 and D3 and the serotonin receptor 5-HT2A with high affinity.
Blonanserin Impurities and Synthesis
Blonanserin synthesis1 is a meticulously controlled process to ensure drug purity and efficacy. Despite stringent measures, impurities may arise, potentially compromising drug quality. Techniques such as chromatography and recrystallization can minimize impurities. Continuous monitoring and adherence to strict quality standards are integral to the synthesis of Blonanserin, guaranteeing its safety and effectiveness.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Blonanserin impurities, which includes Blonanserin C TFA and genotoxic N-Nitroso desethyl Blonanserin impurity. The CoA is from a cGMP-compliant analytical facility and encompasses complete characterization data such as 1H NMR, 13C NMR, IR, MASS2, and HPLC purity. On request, we provide additional data like 13C-DEPT and CHN. Daicel Pharma can also prepare any unidentified Blonanserin impurity or degradation product. Furthermore, Daicel Pharma offers highly purified isotope-labeled standards of Blonanserin for BA/BE studies. We provide a complete characterization report upon delivery.
References
FAQ's
References
- Hino, Katsuhiko; Kai, Naoki; Sakamoto, Masato; Kon, Tatsuya; Oka, Makoto; Furakawa, Kiyoshi; Ochi, Yoshiaki, 2-(1-piperazinyl)-4-phenylcycloalkanopyridine derivatives, processes for the production thereof, and pharmaceutical composition containing the same, EP385237B1, Sep 5, 1990, Dainippon Pharmaceutical Co., Ltd., Japan
- Hattori, Hideki; Iwai, Masae; Ogawa, Tadashi; Mizutani, Yoko; Ishii, Akira; Suzuki, Osamu; Seno, Hiroshi, Simple analysis of blonanserin, a novel antipsychotic agent, in human plasma by GC-MS, Forensic Toxicology, Volume: 28, Issue: 2, Pages: 105-109, 2010, DOI: (10.1007/s11419-010-0090-1)
Frequently Asked Questions
Can Blonanserin impurities affect the therapeutic efficacy of the drug?
Some impurities may alter the pharmacological activity of Blonanserin, leading to reduced efficacy or unexpected side effects.
Are stability studies conducted to assess the long-term effects of Blonanserin impurities?
Stability studies help to evaluate the long-term effects of impurities on the stability and shelf-life of Blonanserin under various storage conditions.
How frequently should impurity levels in Blonanserin be monitored during synthesis?
Impurity levels in Blonanserin should be monitored regularly throughout the synthetic process to ensure compliance with regulatory standards and maintain product quality.
Can Blonanserin impurities affect drug stability over time?
Impurities can contribute to the degradation of Blonanserin over time, leading to reduced stability and shelf-life of the drug product.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.