LOAD MORE
You're viewed 9 of 12 products
Daicel Pharma synthesizes high-quality Bendamustine impurities, including Bendamustine Glycerol Ester Impurity, Bendamustine Isopropyl Ester, Bendamustine HP-1 Impurity, Bendamustine Dihyroxy Impurity, Bendamustine N-Alkylated Impurity, Bendamustine USP Related Compound H and so on. These impurities are essential for evaluating the quality, stability, and safety of Bendamustine, which is an active pharmaceutical ingredient. Additionally, Daicel Pharma offers a customized synthesis of Bendamustine impurities for global delivery to meet the specific needs of our customers.
Bendamustine [CAS: 16506-27-7] is an alkylating agent that treats chronic lymphocytic leukemia and non-Hodgkin lymphoma. As a bifunctional mechlorethamine derivative, Bendamustine acts as an alkylating agent and an antimetabolite, providing dual therapeutic capabilities.
Bendamustine is a medication to treat chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL) that have not responded to treatment with rituximab or rituximab-containing regimens. Bendamustine is available under several trade names, including Belrapzo, Bendeka, Treanda, and Vivimusta.
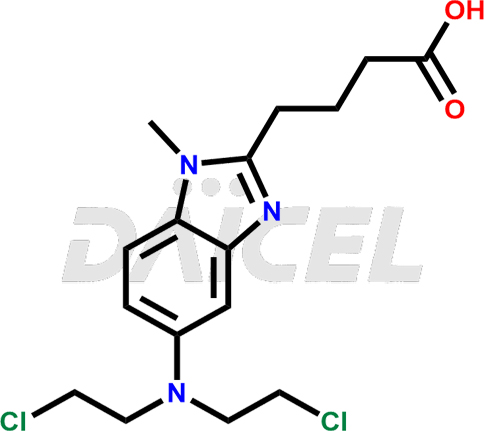
The chemical name of Bendamustine is 5-[Bis(2-chloroethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid. Its chemical formula is C16H21Cl2N3O2, and its molecular weight is approximately 358.3 g/mol.
Bendamustine is a bifunctional bischlorethamine derivative that dissociates into electrophilic alkyl groups that covalently bind with electron-rich nucleophilic moieties leading to cell death.
During the synthesis1 and storage of Bendamustine, impurities that form include degradation products and residual solvents. These impurities can affect the drug’s safety and efficacy and harm patients. So, it is essential to control and monitor these impurities through proper analytical methods and quality control measures to ensure the drug’s purity, potency, and safety for clinical use. Regular testing of the drug substance and drug product can help identify and quantify any impurities and ensure they remain within acceptable limits.
Daicel Pharma provides a Certificate of Analysis (CoA) for Bendamustine impurity standards, including Bendamustine Glycerol Ester Impurity, Bendamustine Isopropyl Ester, Bendamustine HP-1 Impurity, Bendamustine Dihyroxy Impurity, Bendamustine N-Alkylated Impurity, Bendamustine USP Related Compound H and so on. The CoA is generated from a cGMP-compliant analytical facility and includes comprehensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We can also provide additional characterization data like 13C-DEPT and CHN upon request. Daicel Pharma is capable of creating unknown Bendamustine impurities or degradation products. Each delivery has a complete characterization report.
Residual solvents in Bendamustine are impurities that linger after the synthetic process and can be harmful if present in high amounts.
The control of process-related impurities in Bendamustine is possible by using high-quality starting materials, optimizing reaction conditions, and implementing the correct purification steps.
Methanol or DMSO are solvents used in analyzing many impurities in Bendamustine.
Bendamustine impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.