Atomoxetine Impurities
General Information
Atomoxetine Impurities and Atomoxetine
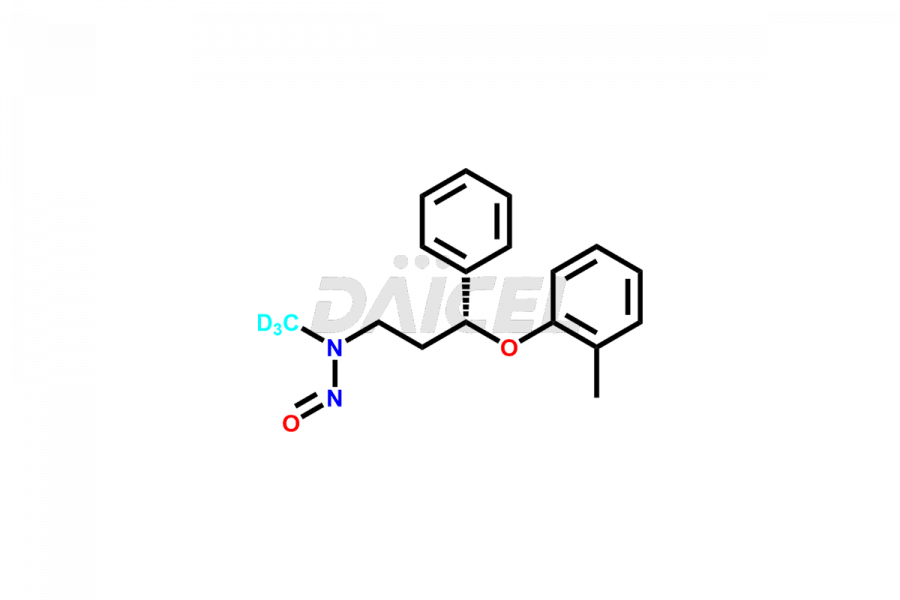
Daicel Pharma offers high-quality Atomoxetine impurities, N-desmethyl-4-hydroxy atomoxetine (HCl Salt), N-Nitroso atomoxetine (Mixture of isomers) and N-Nitroso Atomoxetine-d3. These are vital for evaluating Atomoxetine quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Atomoxetine impurities and ensures their worldwide delivery.
Atomoxetine [CAS: 83015-26-3] treats attention deficit hyperactivity disorder (ADHD) and cognitive disengagement syndrome in adults. It acts as an inhibitor of adrenergic uptake, an antidepressant, and is a xenobiotic. It falls under the category of aromatic ethers and secondary amines.
Atomoxetine: Use and Commercial Availability
Atomoxetine is a non-stimulant medicine approved for treating attention-deficit hyperactivity disorder (ADHD). It is available as the hydrochloride salt of atomoxetine. It is made and commercialized under the trademark Strattera by Eli Lilly and Company. Atomoxetine is for managing ADHD in both adults and children aged six and above. While its FDA approval is solely for ADHD treatment, it is occasionally employed off-label for managing treatment-resistant depression in adult patients.
Atomoxetine Structure and Mechanism of Action
The chemical name of Atomoxetine is (-)-N-methyl-3-phenyl-3-(o-tolyloxy)-propylamine hydrochloride. The chemical formula for Atomoxetine is C17H21NO, and its molecular weight is approximately 255.35g/mol.
The exact mechanism of action of Atomoxetine is unknown. However, it is related to selective inhibition of the pre-synaptic norepinephrine transporter.
Atomoxetine Impurities and Synthesis
Atomoxetine may contain impurities that can influence its safety and efficacy. These impurities form during the synthetic process1, storage, or drug degradation. Therefore, to ensure the safety and quality of Atomoxetine, these impurities must be controlled and monitored throughout the drug’s lifecycle, from production to patient administration.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Atomoxetine impurities, which includes N-desmethyl-4-hydroxy atomoxetine (HCl Salt), and N-Nitroso atomoxetine (Mixture of isomers). This CoA is from a cGMP-compliant analytical facility and encompasses complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. On request, we provide additional data like 13C-DEPT and CHN. Daicel Pharma can prepare any unidentified Atomoxetine impurity or degradation product. Daicel Pharma offers N-Nitroso Atomoxetine-d3, a deuterium-labeled Atomoxetine standard used in BA/BE studies. We give a complete characterization report on delivery.
References
FAQ's
References
- Foster, Bennie Joe; Lavagnino, Edward Ralph, 3-aryloxy-3-phenylpropylamines, EP52492, May 26, 1982, Eli Lilly and Co., United States
- Zhu, Hao-Jie; Wang, Jun-Sheng; Donovan, Jennifer L.; DeVane, C. Lindsay; Gibson, Bryan B.; Markowitz, John S., Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 846, Issue: 1-2, Pages: 351-354, 2007, DOI: (10.1016/j.jchromb.2006.08.019)
Frequently Asked Questions
What is the role of impurity profiling in the synthesis of Atomoxetine?
Impurity profiling helps understand the nature and quantity of impurities in pharmaceuticals, which is crucial for the safety and efficacy of the drug.
What are the steps to take if the level of impurities in Atomoxetine exceeds the acceptable limit?
If the level of impurities in Atomoxetine exceeds the acceptable limit, the particular batch is discarded, and an investigation identifies the source of the impurities.
How is an unknown impurity in Atomoxetine identified?
An unknown impurity identification is possible using the HPLC technique during the commercial synthesis of Atomoxetine.
What are the uses of Atomoxetine impurities reference standards?
Atomoxetine impurities reference standards can help in pharmaceutical research, product development, ANDA and DMF filing, quality control (QC), method validation, and stability studies.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.