LOAD MORE
You're viewed 9 of 13 products
Daicel Pharma synthesizes high-quality Arformoterol impurities, (R)-4-(2-aminopropyl)phenol, A-Amino dimer impurity, A-Hydroxy diol impurity, AC Nitro compound, AC-Amino compound, O-Formyl N-Acetyl AC-Amino, and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Arformoterol. Moreover, Daicel Pharma offers custom synthesis of Arformoterol impurities and delivers them globally.
Arformoterol [CAS: 67346-49-0] is (R, R)-enantiomer of Formoterol and is a selective beta2-adrenergic bronchodilator. It alleviates the symptoms of chronic obstructive pulmonary disease (COPD), a respiratory disorder that makes normal breathing difficult.
Arformoterol is used to maintain the treatment of bronchoconstriction in patients suffering from a chronic obstructive pulmonary disease (COPD), which includes conditions such as chronic bronchitis and emphysema. It is usually taken through inhalation as a direct-acting sympathomimetic and bronchodilator to help alleviate the symptoms of COPD. Arformoterol is available under the trade names of Arformoterol Tartrate and Brovana.
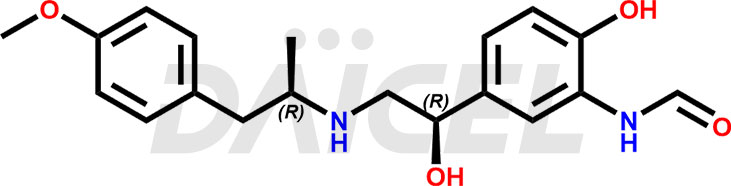
The chemical name of Arformoterol is N-[2-Hydroxy-5-[(1R)-1-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formamide. Its chemical formula isC19H24N2O4, and its molecular weight is approximately 344.4 g/mol.
Arformoterol is the (R, R) enantiomer of Formoterol which has two-fold greater potency than racemic Formoterol. It is a long-acting beta2-adrenergic receptor agonist that increases intracellular cyclic AMP levels causing relaxation of bronchial smooth muscle.
Arformoterol impurities form during the synthesis1,2 or storage of the drug product. Its common impurities include related compounds, degradation products, and process impurities. They can also occur through the use of certain raw materials or intermediates during the manufacturing process. Strict quality control measures and analytical methods are necessary to ensure the purity and safety of Arformoterol for patient use.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Arformoterol impurity standards, (R)-4-(2-aminopropyl)phenol, A-Amino dimer impurity, A-Hydroxy diol impurity, AC Nitro compound, AC-Amino compound, O-Formyl N-Acetyl AC-Amino, and more. The CoA includes characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity3. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Arformoterol impurity or degradation product.
Arformoterol can undergo various degradation pathways that lead to the formation of degradation impurities. These pathways include oxidation, deamination, and hydrolysis.
Arformoterol impurities are detected through various analytical methods such as High-Performance Liquid Chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), etc.
Methanol is a solvent used in analyzing Arformoterol impurities.
Arformoterol impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.