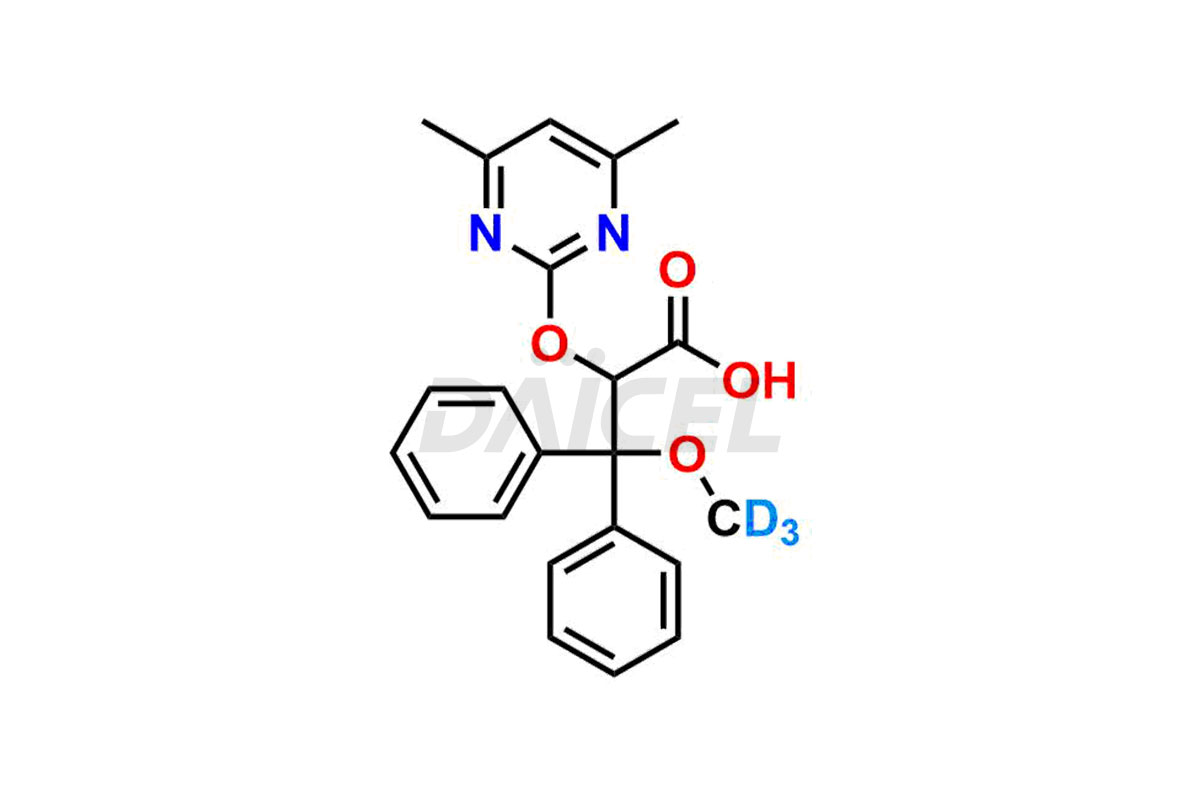
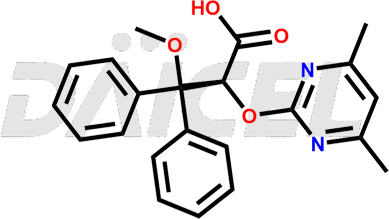
Ambrisentan
General Information
Ambrisentan Impurities and Ambrisentan
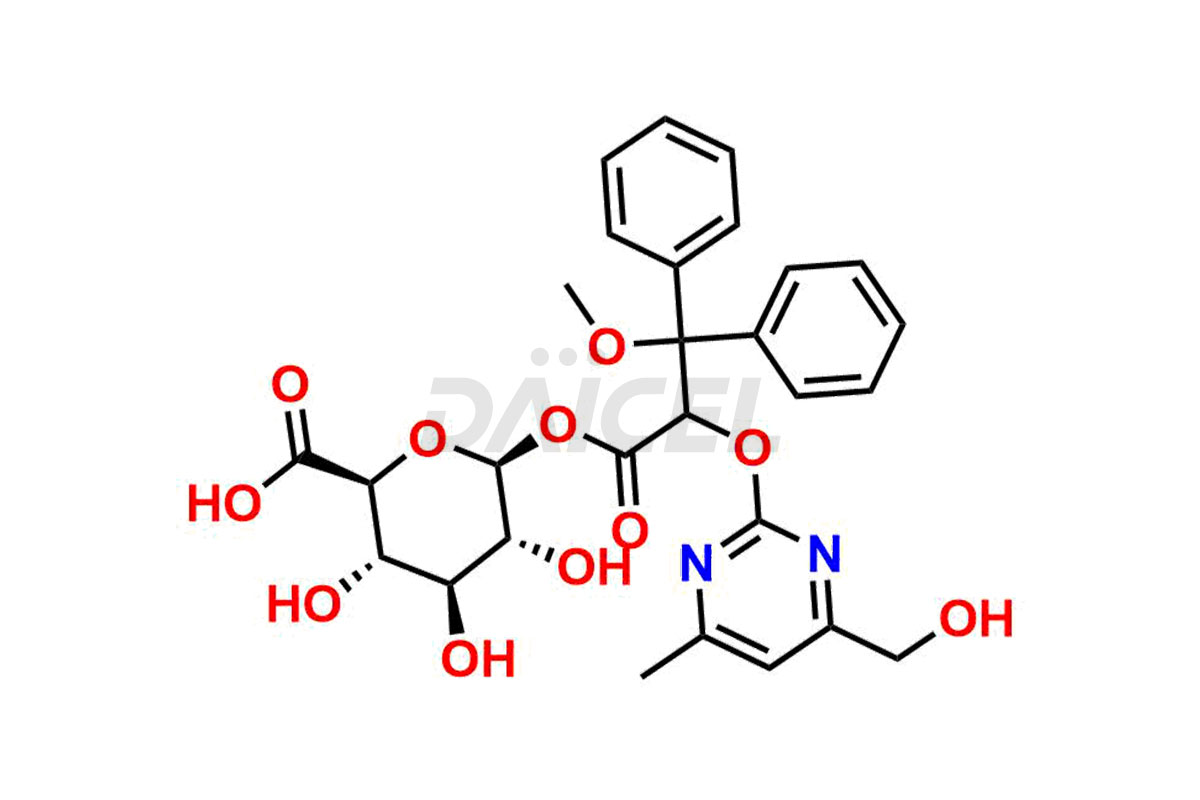
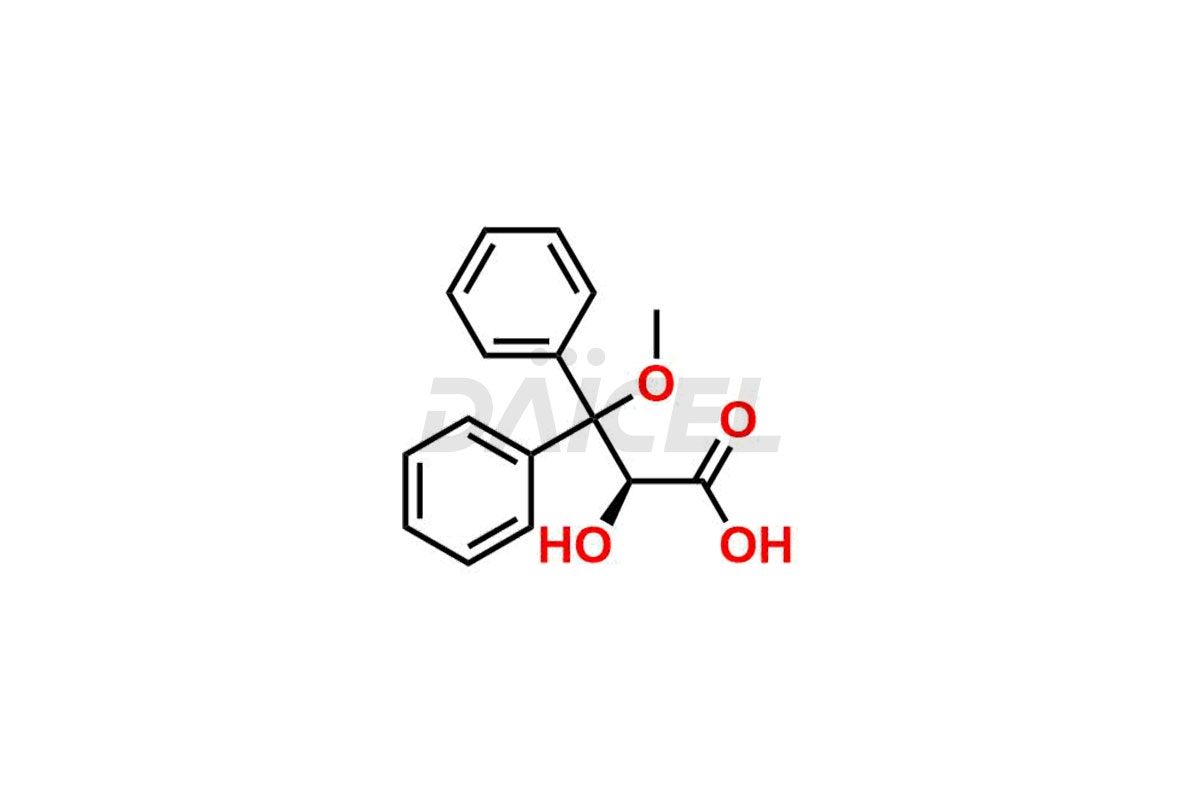
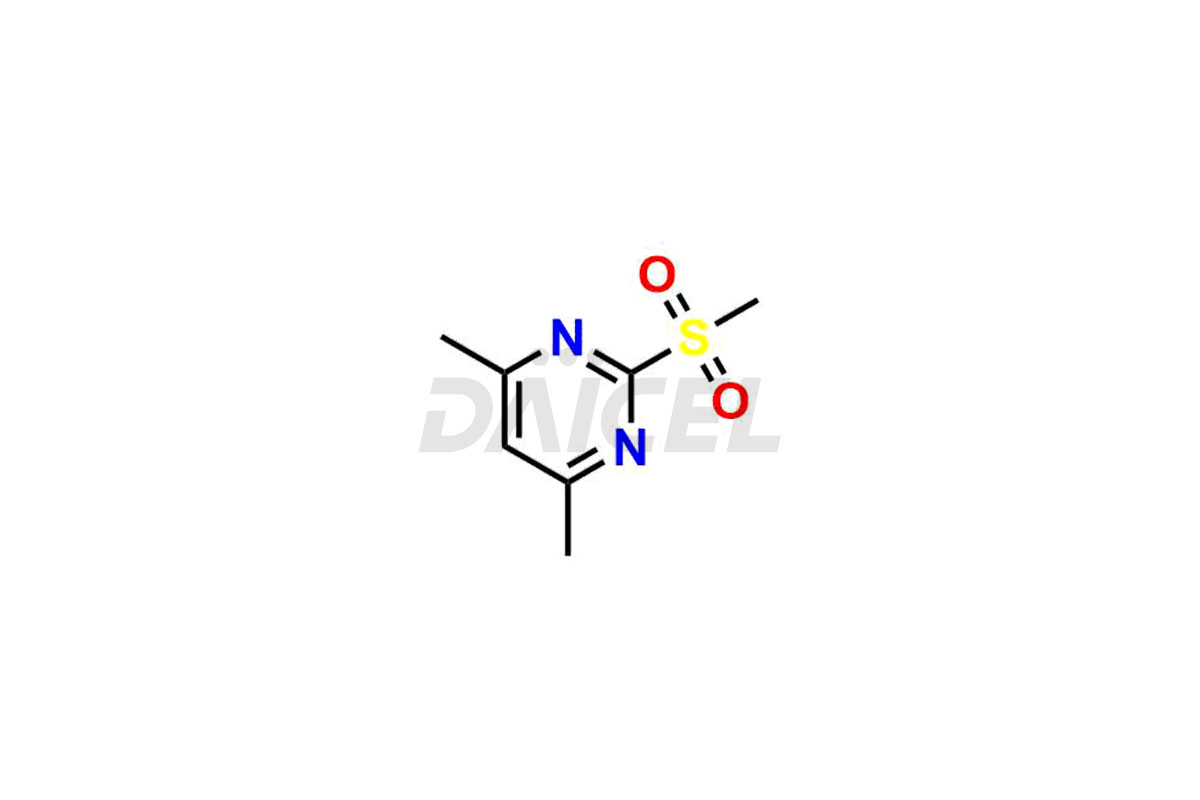
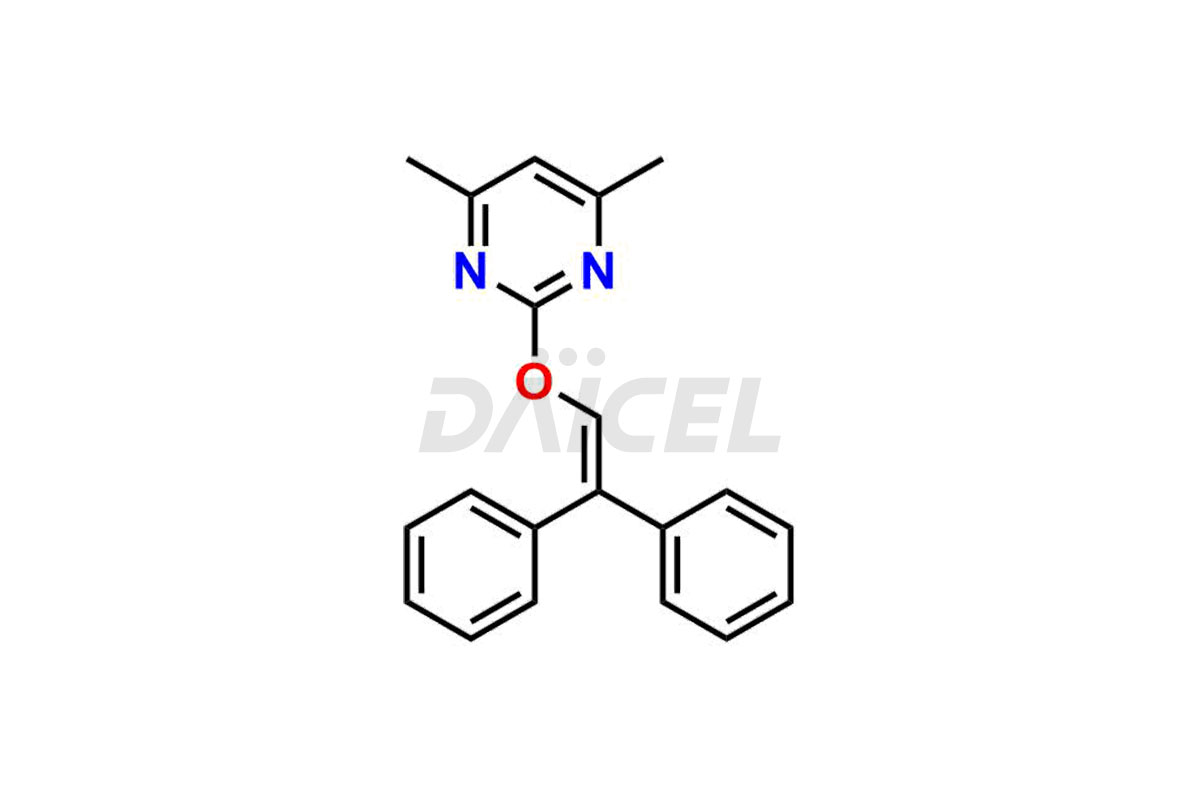
Daicel Pharma is a reliable source for synthesizing high-quality Ambrisentan impurities, specifically (R)-Ambrisentan, 4-Hydroxymethyl Ambrisentan glucuronide, Ambrisentan Impurity A, Ambrisentan Impurity C, and Ambrisentan Impurity D. These impurities help in assessing the quality, stability, and safety of the active pharmaceutical ingredient, Ambrisentan. Daicel Pharma also offers a custom synthesis of Ambrisentan impurities, which can be shipped worldwide to meet customers’ unique requirements.
Ambrisentan [CAS: 177036-94-1] is an oral medication that targets the endothelin type-A (ETA) receptor by blocking it. It treats pulmonary arterial hypertension (PAH).
Ambrisentan: Use and Commercial Availability
Ambrisentan is a medicine for treating patients with pulmonary arterial hypertension (PAH) and idiopathic(primary)pulmonary arterial hypertension (IPAH). It can combine with tadalafil to minimize the risks of disease progression and hospitalization and enhance exercise ability. Letairis is the brand name under which Ambrisentan is available.
Ambrisentan Structure and Mechanism of Action
The chemical name of Ambrisentan is (2S)-2-(4,6-Dimethylpyrimidin-2-yl)oxy-3-methoxy-3,3-diphenylpropanoic acid. Its chemical formula is C22H22N2O4, and its molecular weight is approximately 378.4 g/mol.
Ambrisentan is an ETA receptor antagonist with high selectivity for ETA, whose activity includes vasoconstriction and cell proliferation.
Ambrisentan Impurities and Synthesis
Impurities form during the manufacturing1 of Ambrisentan due to the use of starting materials, intermediates, or reagents. These impurities may decrease the drug’s efficacy and pose risks to patients. As a result, it’s critical to control and monitor the impurity levels.
Daicel Pharma provides a Certificate of Analysis (CoA) for Ambrisentan impurity standards, which includes (R)-Ambrisentan, 4-Hydroxymethyl Ambrisentan glucuronide, Ambrisentan Impurity A, Ambrisentan Impurity C, and Ambrisentan Impurity D. The CoA is from an analytical facility that adheres to current Good Manufacturing Practices (cGMP) and includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization data such as 13C-DEPT and CHN on request. Daicel Pharma can also generate unknown Ambrisentan impurities or degradation products and provide labeled compounds to assess the effectiveness of Ambrisentan. Further, Daicel Pharma offers Ambrisentan-D3, a deuterium-labeled Ambrisentan standard used in bio-analytical research, such as BA/BE studies. A complete characterization report is part of each delivery.
References
FAQ's
References
- Riechers, Hartmut; Klinge, Dagmar; Amberg, Wilhelm; Kling, Andreas; Mueller, Stefan; Baumann, Ernst; Rheinheimer, Joachim; Vogelbacher, Uwe Josef; Wernet, Wolfgang; Unger, Liliane; et al, Carboxylic acid derivatives, their preparation and use, Abbott Gmbh & Co. KG, Germany, US7109205B2, September 19, 2006
- Ramisetti, Nageswara Rao; Kuntamukkala, Ramakrishna, LC-MS/MS characterization of forced degradation products of ambrisentan: development and validation of a stability-indicating RP-HPLC method, New Journal of Chemistry, Volume: 38, Issue: 7, Pages: 3050-3061, 2014
Frequently Asked Questions
Why is it vital to analyze Ambrisentan impurities?
Analyzing impurities in Ambrisentan is critical to ensure the drug's safety and effectiveness. They can affect drug quality, stability, and safety reducing its efficacy or causing harm to patients. Analyzing impurities can help identify their sources, enabling manufacturers to control their formation.
What steps can manufacturers take to control impurity levels in Ambrisentan?
Manufacturers can take various steps to control impurity levels in Ambrisentan, including using high-quality starting materials, optimizing reaction conditions, implementing effective purification techniques, and monitoring impurity levels throughout the manufacturing process.
Which solvent helps in the analysis of Ambrisentan impurities?
Methanol is a solvent that helps in analyzing many impurities in Ambrisentan.
What are the temperature conditions required to store Ambrisentan impurities?
Ambrisentan impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.