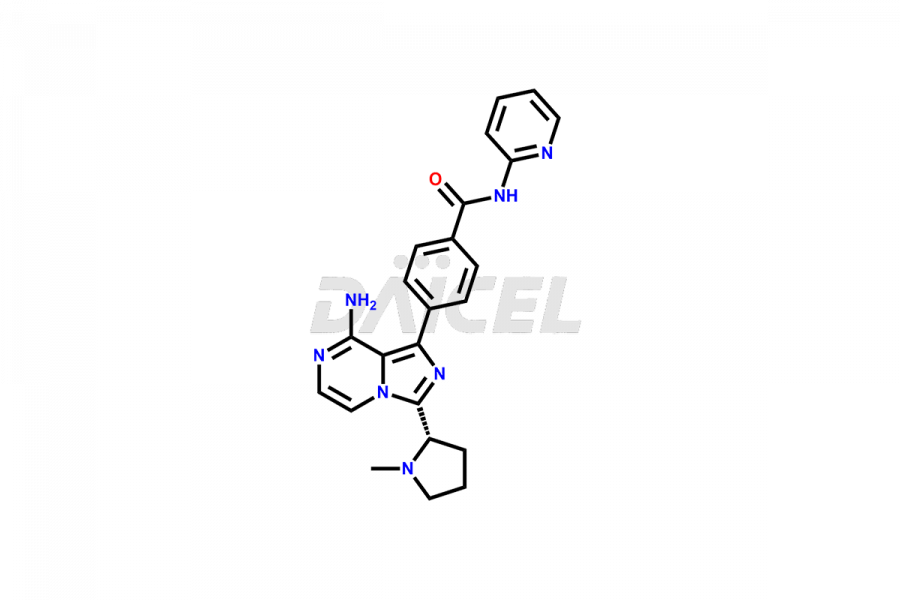
Acalabrutinib
References
- Barf, Tjeerd A.; Jans, Christiaan Gerardus Johannes Maria; De Man, Adrianus Petrus Antonius; Oubrie, Arthur A.; Raaijmakers, Hans C. A.; Rewinkel, Johannes Bernardus Maria; Sterrenburg, Jan-Gerard; Wijkmans, Jacobus C. H. M., 4-Imidazopyridazin-1-Yl-Benzamides And 4-Imidazotriazin-1-Yl-Benzamides As BTK-Inhibitors, MSD Oss B.V., Netherlands, EP2734522B1, July 11, 2012
- Krishna, G. Atchutarama; Srinivasarao, P.; Patrudu, T. Benarji; Chidanandaswamy, R., Development and validation of novel HPLC bioanalytical analysis method for acalabrutinib: an anticancer drug in human plasma, Asian Journal of Chemistry, Volume: 32, Issue: 10, Pages: 2606-2610, 2020
Frequently Asked Questions
Why is it vital to control Acalabrutinib impurities?
It is vital to control the impurities in Acalabrutinib because they can affect the drug's safety, efficacy, and stability. In addition, regulatory agencies require the identification and control of impurities to ensure the quality of the drug.
What are the analytical techniques used to detect and quantify Acalabrutinib impurities?
The analytical techniques used to detect and quantify the impurities in Acalabrutinib include high-performance liquid chromatography (HPLC), RP-HPLC, liquid chromatography-mass spectrometry (LC-MS), etc.
How are Acalabrutinib impurities controlled during drug development and manufacturing?
Acalabrutinib impurities are controlled through various measures, such as optimizing the synthetic process, conducting appropriate analytical testing, and implementing strict quality control measures during manufacturing.
What are the temperature conditions required to store Acalabrutinib impurities?
Acalabrutinib impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.