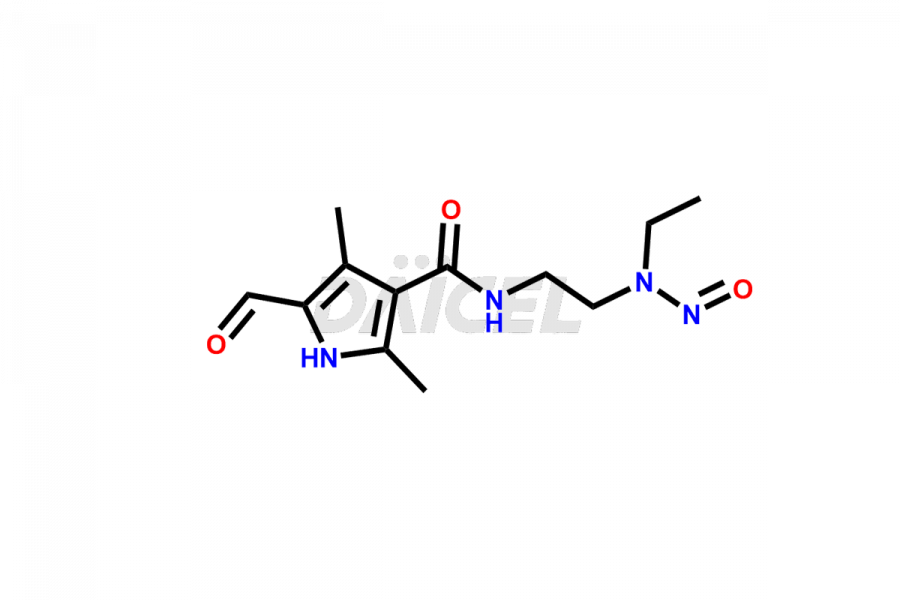
N-(2-(ethyl(nitroso)amino)ethyl)-5-formyl-2,4-dime...
- CAT Number DCTI-C-002468
- CAS NUMBER NA
- MOLECULAR FORMULA C12H18N4O3
- MOLECULAR WEIGHT 266.3
tert-butyl 4-((2-(ethyl(nitroso)amino)ethyl)carbam...
- CAT Number DCTI-C-002469
- CAS NUMBER NA
- MOLECULAR FORMULA C16H26N4O4
- MOLECULAR WEIGHT 338.41
N-(2-aminoethyl)-N-ethylnitrous amide
- CAT Number DCTI-C-002586
- CAS NUMBER NA
- MOLECULAR FORMULA C4H11N3O (Free Base); C6H11F3N3O2 (TFA Salt)
- MOLECULAR WEIGHT 117.15 (Free Base); 214.17 (TFA Salt)