Deuterated Suplatast impurity D-1 (M-1)
- CAT Number DCTI-C-002852
- CAS NUMBER NA
- MOLECULAR FORMULA C14H14D5NO4
- MOLECULAR WEIGHT 270.34
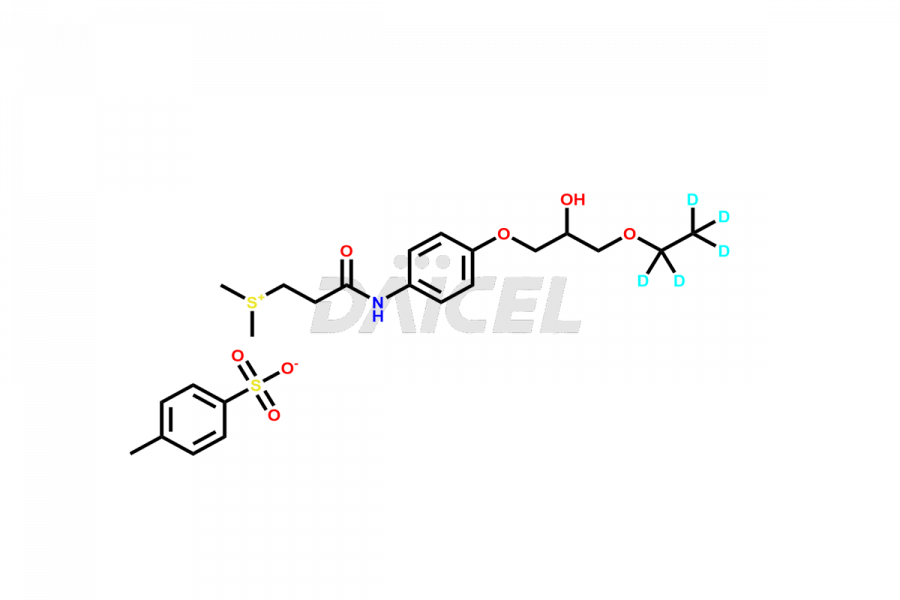
Deuterated Suplatast Tosilate
- CAT Number DCTI-A-000332
- CAS NUMBER NA
- MOLECULAR FORMULA C16H21D5NO4S+ (without salt); C23H28D5NO7S2 (p-toluenesulfonate salt)
- MOLECULAR WEIGHT 333.48 (without salt); 504.67 (p-toluenesulfonate salt)