GLP-1 peptide therapeutics such as Liraglutide, Semaglutide, and Tirzepatide are administered chronically and often at high cumulative doses, making immunogenicity risk a critical consideration during development and regulatory review. While the active drug substance is carefully optimized to minimize immune responses, peptide-related impurities may significantly alter immunogenic potential, even when present at low levels.
This blog examines how impurities in GLP-1 peptide drugs contribute to immunogenicity risk, and how regulatory frameworks and analytical strategies are designed to mitigate this risk.
Why Immunogenicity Matters for GLP-1 Peptide Drugs
Peptide-based therapeutics inherently carry a higher immunogenic risk than small molecules due to their size, sequence complexity, and structural similarity to endogenous proteins. For GLP-1 analogs, repeated administration increases the likelihood that the immune system may recognize structural variants or modified peptides as foreign.
Potential clinical consequences include:
-
-
- Development of anti-drug antibodies (ADAs)
- Reduced therapeutic efficacy
- Altered pharmacokinetics
- Injection-site reactions or systemic hypersensitivity
-
Regulators therefore place strong emphasis on impurity profiles that could increase immunogenic potential beyond that of the Reference Listed Drug (RLD)
Impurity Attributes That Increase Immunogenic Risk
Not all impurities pose equal immunogenic concern. Risk is influenced by structure, novelty, and exposure.
- Sequence Variants and Truncated Peptides
Deletion, insertion, and truncated impurities introduce new peptide termini or altered epitopes. These changes may expose sequences not present in the native GLP-1 analog, increasing the likelihood of immune recognition. - D-Amino Acid and Isomeric Impurities
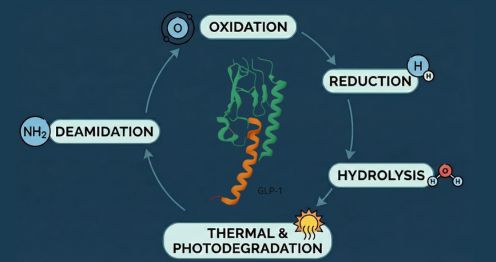
Racemization during synthesis can produce D-amino acid–containing peptides. As these are not typically present in human proteins, they may increase the probability of immune recognition. - Oxidative and Deamidated Impurities
Oxidation or deamidation can subtly change peptide conformation, potentially altering antigen processing and presentation pathways. - Aldehyde and Excipient Adducts
Drug product–related impurities such as formaldehyde or acetaldehyde adducts introduce non-native chemical groups, which are well-recognized immunogenic triggers.
Regulatory Expectations for Immunogenicity Control
During ANDA review of peptide drugs, regulatory agencies evaluate impurity-related immunogenic risk using a comparability-based and risk driven approach:
- Impurities present in the RLD at similar levels are generally acceptable.
- New impurities absent in the RLD are expected to be at ≤0.1% and may require additional scientific justification based on structure and exposure.
- Unknown impurities must be controlled at ≤0.1% in every batch.
- Immunogenicity risk should be justified using structural, analytical, and comparative data rather than clinical assumptions alone.
As a result, analytical characterization plays a central role in regulatory decision-making.
Role of Analytical Characterization in Immunogenicity Assessment
Comprehensive impurity characterization directly supports immunogenicity risk evaluation by enabling:
Structural Confirmation
High-resolution mass spectrometry (HRMS) and amino acid sequencing identify whether an impurity introduces new epitopes or altered peptide backbones.
Purity and Potency Determination
Accurate potency assignment ensures that impurity levels are not underestimated due to residual salts, solvents, or counter ions.
Comparability to RLD Profiles
Orthogonal chromatographic and HRMS data allow side-by-side comparison of impurity profiles between generic and innovator products. Without fully characterized impurity standards, immunogenicity risk assessments remain incomplete and vulnerable to regulatory challenge.
Stability-Induced Immunogenic Risk
Stability studies are particularly important for immunogenicity evaluation, as certain impurities may not be present at release but can emerge over shelf life. Oxidative species, deamidated variants, and cyclic degradation products often increase under accelerated or stressed conditions.
Monitoring impurity evolution during stability testing helps ensure that immunogenic risk does not increase over time, especially for long-term therapies like GLP-1 analogs.
Importance of Certified Impurity Standards
Certified GLP-1 impurity standards enable:
- Accurate quantification of low-level immunogenic impurities
- Validation of sensitive analytical methods
- Reliable stability trend analysis
- Strong scientific justification in regulatory submissions
These standards help bridge analytical with immunogenicity risk assessment.
Daicel Pharma Standards’ Contribution
Daicel Pharma Standards provides an extensive portfolio of fully characterized GLP-1 peptide impurity standards, including sequence variants, oxidative species, deamidated forms, truncated peptides, and drug product–related adducts.
By supporting precise impurity identification and quantification, these standards help pharmaceutical developers proactively assess and control immunogenicity risk throughout the GLP-1 product lifecycle.
Conclusion
Immunogenicity risk in GLP-1 peptide therapeutics is closely linked to impurity structure, origin, and evolution over time. Regulatory agencies expect manufacturers to demonstrate not only low impurity levels, but also a deep understanding of how those impurities may affect patient safety. A scientifically rigorous impurity characterization and control strategy supported by certified impurity standards remains essential for minimizing immunogenic risk and ensuring long-term success of GLP-1 peptide drugs.

Write a comment
Your email address will not be published. All fields are required