GLP-1 peptide drug therapeutics have revolutionized the treatment of type 2 diabetes and obesity due to their ability to regulate blood glucose levels by promoting insulin secretion, inhibiting glucagon release, and delaying gastric emptying. Critical structural modifications, such as C-terminal amidation and the presence of histidine at position 7, enhance their therapeutic efficacy and extend their half-life. Among these analogs, Liraglutide, Semaglutide, and Tirzepatide are prominent examples. However, understanding their degradation pathways and impurity formation is essential for ensuring their safety, efficacy, and stability.
Overview of GLP-1 peptide drugs:
- Liraglutide: A daily injectable GLP-1 analog engineered to resist DPP-4 degradation. Its fatty acid modification enhances albumin binding, prolonging its half-life and improving therapeutic outcomes.
- Semaglutide: Offered in both injectable and oral forms, Semaglutide boasts greater potency and a much longer half-life (~170 hours) than Liraglutide, making it a favored choice for weight management.
- Tirzepatide: This dual agonist of GLP-1 and GIP delivers superior glycemic control and weight reduction, setting it apart as the latest innovation in this drug class.
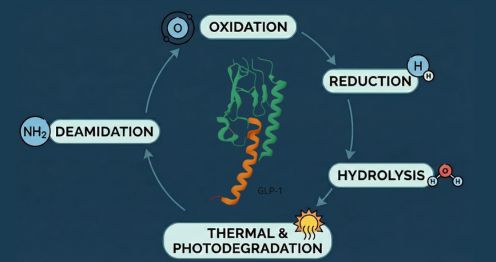
Degradation Pathways of GLP-1 peptide drugs:
GLP-1 peptide drugs are vulnerable to degradation, leading to impurity formation that affects their safety, efficacy, and immunogenicity.
- Hydrolysis: Peptide bonds degrade in aqueous conditions, producing inactive fragments. Factors like pH, temperature, and ionic content influence hydrolysis rates.
- Oxidation: Residues such as tryptophan, tyrosine, and histidine are prone to oxidation, forming impurities that may elicit immune responses. Environmental factors like air, light, and pro-oxidant excipients exacerbate this process.
- Thermal Degradation: Heat accelerates hydrolysis and oxidation, leading to peptide aggregation and truncated sequences that undermine drug performance.
- Photodegradation: Exposure to light triggers structural damage to peptides. Proper storage minimizes this risk.
- Deamidation: Asparagine and glutamine residues undergo deamidation, creating acidic variants that alter stability and pharmacokinetics.
Impurity Formation in GLP-1 peptide drugs
Liraglutide: Liraglutide, a 31-amino acid peptide, is prone to degradation and impurity formation due to its structural features. It contains glycine and aspartic acid residues, along with vulnerable peptide bonds, making it susceptible to bond cleavage and hydrolysis. Glutamine can undergo deamidation, while aspartic acid may isomerize to isoaspartic acid, affecting stability. The tryptophan residue is at risk of oxidative degradation, forming impurities like kynurenine under photodegradation conditions. Tyrosine and histidine are also prone to oxidation. Thermal degradation can cleave amide bonds, producing shorter peptides like 2,5-diketopiperazines. Additionally, aldehyde reagents used in formulation increase the risk of impurity formation.
Examples of impurities:
[1-28]-Liraglutide, [3-31]-Liraglutide, [4-31]-Liraglutide, [5-31]-Liraglutide, (6-31)-Liraglutide, [7-31]-Liraglutide, (9-31)-Liraglutide, [10-31]-Liraglutide, [11-31]-Liraglutide, [12-31]-Liraglutide, [14-31]-Liraglutide, Linear Liraglutide, Iso-Asp-Liraglutide, Trp(O)-Liraglutide, Kyn(25)-Liraglutide , Glu(17)-Liraglutide, Formaldehyde adduct-Liraglutide, Acetaldehyde-Adduct-Liraglutide, Propionaldehyde adduct-Liraglutide.
Semaglutide: Structurally similar to Liraglutide, Semaglutide incorporates α-aminoisobutyric acid (Aib) and has a longer linker at Lys20. The degradation pathways for Semaglutide mirror those of Liraglutide, but its structural modifications enhance stability.
Examples of impurities:
[1-29]-Semaglutide, [3-31]-Semaglutide, [4-31]-Semaglutide, [5-31]-Semaglutide, [9-31]-Semaglutide, [12-31]-Semaglutide, [14-31]-Semaglutide, Linear Semaglutide, Glu(17)-Semaglutide, γ-Glu(3)-Semaglutide, γ-Glu(15)-Semaglutide, Iso-Asp-Semaglutide, Acetaldehyde adduct-Semaglutide
Tirzepatide: With a larger backbone of 39 amino acids and a dual agonistic mechanism, Tirzepatide shares similar degradation pathways with Liraglutide and Semaglutide. Its unique sequence introduces additional proline residues, reducing susceptibility to specific degradation reactions.
Examples of impurities:
[1-31]-Tirzepatide, Tirzepatide Acid, Glu(19)-Tirzepatide, Glu(24)-Tirzepatide, [β-Asp-9, β-Asp-15]-Tirzepatide, Endo-Pro(38)-Tirzepatide, [Des-AEEA4′]-Tirzepatide, [Endo-AEEA4′]-Tirzepatide, [D-Ser8]-Tirzepatide, [D-Ser32]-Tirzepatide, [Kyn25]-Tirzepatide, Trp(O)-Tirzepatide, Cycli-Tyr-Tirepatide
Daicel Pharma Standards Contribution
Daicel Pharma Standards provides a comprehensive portfolio of fully characterized GLP-1 peptide drug impurity standards, including degradation-related impurities for Liraglutide, Semaglutide, and Tirzepatide. These standards play a critical role in method development, regulatory submissions, and stability studies, strengthening Daicel Pharma Standards’ position as a trusted partner in accelerating peptide drug development. By enabling precise and accurate impurity assessment, Daicel Pharma Standards supports the pharmaceutical industry’s efforts to advance safe, effective, and innovative GLP-1 peptide drugs for the management of diabetes and obesity.
Daicel Pharma Standards stock list of GLP-1 peptide drug impurities:
| S. No. | Liraglutide | Semaglutide | Tirzepatide |
| isomeric impurities | isomeric impurities | isomeric impurities | |
| 1 | D-His(1)-Liraglutide | D-His(1)-Semaglutide | D-Tyr(1)-Tirzepatide |
| 2 | D-Glu(3)-Liraglutide | D-Glu(3)-Semaglutide | [D-Glu-3]-Tirzepatide |
| 3 | D-Thr(5)-Liraglutide | D-Thr(5)-Semaglutide | [D-Phe6]-Tirzepatide |
| 4 | D-allo-Thr(5)-Liraglutide | D-Allo-Thr(5)-Semaglutide | [D-Ser8]-Tirzepatide |
| 5 | D-Thr(7)-Liraglutide | D-Phe(6)-Semaglutide | [D-Asp9]-Tirzepatide |
| 6 | D-allo-Thr(7)-Liraglutide | D-Ser(8)-Semaglutide | [D-Ser11]-Tirzepatide |
| 7 | D-Ser(8)-Liraglutide | D-Asp(9)-Semaglutide | [D-Leu14]-Tirzepatide |
| 8 | D-Asp(9)-Liraglutide | D-Iso-Asp(9)-Semaglutide | [D-Asp15]-Tirzepatide |
| 9 | D-Val(10)-Liraglutide | D-Val(10)-Semaglutide | [D-Gln19]-Tirzepatide |
| 10 | D-Ser(11)-Liraglutide | D-Ser(11)-Semaglutide | [D-γ-Glu-Side chain]-Tirzepatide |
| 11 | D-Ser(12)-Liraglutide | D-Ser(12)-Semaglutide | [D-Phe22]-Tirzepatide |
| 12 | D-Leu(14)-Liraglutide | D-Leu(14)-Semaglutide | [D-Gln24]-Tirzepatide |
| 13 | D-Glu(15)-Liraglutide | D-Glu(15)-Semaglutide | D-Leu(26)-Tirzepatide |
| 14 | D-Gln(17)-Liraglutide | D-Ala(19)-Semaglutide | D-Ser(32)-Tirzepatide |
| 15 | D-Ala(18)-Liraglutide | D-γ-Glu(side chain)-Semaglutide | D-Ser(33)-Tirzepatide |
| 16 | D-γ-Glu(side chain)-Liraglutide | D-Glu(21)-Semaglutide | |
| 17 | D-Glu(21)-Liraglutide | D-Phe(22)-Semaglutide | |
| 18 | D-Phe(22)-Liraglutide | D-Ile(23)-Semaglutide | |
| 19 | D-Ile(23)-Liraglutide | D-Ala(24)-Semaglutide | |
| 20 | D-Ala(24)-Liraglutide | D-Trp(25)-Semaglutide | |
| 21 | D-Trp(25)-Liraglutide | D-Leu(26)-Semaglutide | |
| 22 | D-Leu(26)-Liraglutide | D-Arg(30)-Semaglutide | |
| 23 | D-Arg(28)-Liraglutide | ||
| 24 | D-Arg(30)-Liraglutide | ||
| truncated impurities | truncated impurities | truncated impurities | |
| 25 | [3-31]-Liraglutide | [3-31]-Semaglutide | Des-Tyr(1),Aib(2)-Tirzepatide |
| 26 | [4-31]-Liraglutide | [4-31]-Semaglutide | Fragment (1-20)-Tirzepatide |
| 27 | [5-31]-Liraglutide | [5-31]-Semaglutide | Fragment (1-21)-Tirzepatide |
| 28 | (6-31)-Liraglutide | [7-31]-Semaglutide | Fragment (1-24)-Tirzepatide |
| 29 | [7-31]-Liraglutide | [8-31]-Semaglutide | Fragment (1-25)-Tirzepatide |
| 30 | [8-31]-Liraglutide | [9-31]-Semaglutide | Fragment (1-26)-Tirzepatide |
| 31 | (9-31)-Liraglutide | [10-31]-Semaglutide | Fragment (1-28)-Tirzepatide |
| 32 | [10-31]-Liraglutide | [12-31]-Semaglutide | Fragment (1-32)-Tirzepatide |
| 33 | [11-31]-Liraglutide | [14-31]-Semaglutide | Fragment (3-39)-Tirzepatide |
| 34 | [12-31]-Liraglutide | [1-29]-Semaglutide | Fragment (4-39)-Tirzepatide |
| 35 | [14-31]-Liraglutide | Des(3-6)-Semaglutide | Fragment (5-39)-Tirzepatide |
| 36 | [1-28]-Liraglutide | Des(3-7)-Semaglutide | Fragment (6-39)-Tirzepatide |
| 37 | fragment (21-28) Ile-Liraglutide | Des(3-8)-Semaglutide | Fragment (7-39)-Tirzepatide |
| 38 | fragment (21-28) Leu-Liraglutide | Fragment (8-39)-Tirzepatide | |
| 39 | Fragment (9-39)-Tirzepatide | ||
| 40 | Fragment (10-39)-Tirzepatide | ||
| deletion impurities | deletion impurities | deletion impurities | |
| 41 | Asn(12)-Linear Liraglutide | Linear Semaglutide | Des-Tyr(1)-Tirzepatide |
| 42 | Asn(11)-Linear Liraglutide | Des-His(1)-Semaglutide | [Des-Aib2]-Tirzepatide |
| 43 | Asn(8)-Linear Liraglutide | Des-Aib(2)-Semaglutide | Des-Gly(4)-Tirzepatide |
| 44 | Thr(8)-Linear Liraglutide | Des-Glu(3)-Semaglutide | Des-Thr(5)-Tirzepatide |
| 45 | Glu(9)-Linear Liraglutide | Des-Gly(4)-Semaglutide | Des-Ile(12)-Tirzepatide |
| 46 | Thr(12)-Linear Liraglutide | Des-Thr(5)-Semaglutide | Des-Aib(13)-Tirzepatide |
| 47 | Thr(11)-Linear Liraglutide | Des-Thr(7)-Semaglutide | [Des-Ile17]-Tirzepatide |
| 48 | Des-His(1)-Liraglutide | Des-Ser(8)-Semaglutide | Des-ϒ-Glu Tirzepatide |
| 49 | Des-Ala(2)-Liraglutide | Des-Tyr(13)-Semaglutide | Des-AEEA-Tirzepatide |
| 50 | Des-Gly(4)-Liraglutide | Des-Gly(29)-Semaglutide | Des-Gly(30)-Tirzepatide |
| 51 | Des-Thr(5)-Liraglutide | Des-Gly(31)-Semaglutide | Des-Pro(31)-Tirzepatide |
| 52 | Des-Thr(7)-Lira | Des-AEEA-Semaglutide | Des-Gly(34)-Tirzepatide |
| 53 | Des-Ser(8)-Liraglutide | Des-Pro(38)-Tirzepatide | |
| 54 | Des-Gly(29)-Liraglutide | ||
| 55 | Des-Gly(31)-Liraglutide | ||
| Oxidative impurities | Oxidative impurities | Oxidative impurities | |
| 56 | Trp(O)-Liraglutide | Trp(5-OH)-Semaglutide | Trp(5-OH)-Tirzepatide |
| 57 | Trp(5-OH)-Liraglutide | Trp(2-Oxo)-Semaglutide | Trp(O)25-Tirzepatide |
| 58 | Kyn(25)-Liraglutide | Kyn(25)-Semaglutide | Kyn(25)-TirzepatideLiraglutide |
| 59 | NFK-Tirzepatide | ||
| Process related impurities | Process related impurities | Process related impurities | |
| 60 | N-Ac-Liraglutide | N-Ac-Semaglutide | [β-Asp9]-Tirzepatide |
| 61 | N-Ac-D-His-Liraglutide | Me-His-Semaglutide | [β-Asp15]-Tirzepatide |
| 62 | Iso-Asp-Liraglutide | γ-Glu(3)-Semaglutide | [β-Asp-9, β-Asp-15]-Tirzepatide |
| 63 | α-Glu(side Chain)-Liraglutide | Iso-Asp-Semaglutide | [β-Ala18]-Tirzepatide |
| 64 | Trp(4-hydroxy benzyl)25-Liraglutide | γ-Glu(15)-Semaglutide | [β-Ala21]-Tirzepatide |
| 65 | Trp(Ac)-Liraglutide | Pyro-glu-17-Semaglutide | [β-Ala28]-Tirzepatide |
| 66 | Trp(tBu)25-Liraglutide | α-Glu(side chain)-Semaglutide | [β-Ala-35]-Tirzepatide |
| 67 | Trp(4-hydroxybenzyl)25-Semaglutide | ||
| 68 | Trp(tBu)25-Semagltuide | ||
| Insertion impurities | Insertion impurities | Insertion impurities | |
| 69 | Endo-Thr(7)-Lira | Endo-Aib(2)-Semaglutide | Endo-Gly(4a)-Tirzepatide |
| 70 | Endo-Ala(18)-Liraglutide | Endo-Glu(3)-Semaglutide | Endo-Thr(5)-Tirzepatide |
| 71 | D-His(1)-Endo-Ala(18)-Liraglutide | Endo-Gly(4)-Semaglutide | Endo-AEEA-Tirzepatide |
| 72 | Endo-Ala(24)-Liraglutide | Endo-Thr(5)-Semaglutide | Endo-Tyr(4’a)-Tirzepatide |
| 73 | Endo-Gly(31)-Liraglutide | Endo-Tyr(13)-Semaglutide | Endo-Gly(30a, 30b)-Tirzepatide |
| 74 | Endo-Gly(16)-Semaglutide | Endo-Pro(31)-Tirzepatide | |
| 75 | Endo-Gln(17)-Semaglutide | Endo-Ala(35)-Tirzepatide | |
| 76 | Endo-Ala(19)-Semaglutide | Endo-Pro(38)-Tirzepatide | |
| 77 | Endo-AEEA-Semaglutide | Endo-Ser(39)-Tirzepatide | |
| 78 | Endo-Ile(23)-Semaglutide | ||
| 79 | Endo-Ala(24)-Semaglutide | ||
| 80 | Endo-Leu(26)-Semaglutide | ||
| 81 | Endo-Gly(31)-Semaglutide | ||
| Deamidated impurities | Deamidated impurities | Deamidated impurities | |
| 82 | Glu(17)-Liraglutide | Glu(17)-Semaglutide | Glu(19)-Tirzepatide |
| 83 | D-Glu(17)-Liraglutide | Glu(24)-Tirzepatide | |
| 84 | Tirzepatide Acid | ||
| Drug product impurities | Drug product impurities | Drug product impurities | |
| 85 | Formaldehyde adduct-Liraglutide | His-Cyclic-Semaglutide | Cyclic-Tyr-Tirzepatide |
| 86 | Acetaldehyde-Adduct-Liraglutide | Acetaldehyde adduct-Semaglutide | |
| 87 | Propionaldehyde adduct-Liraglutide |

Write a comment
Your email address will not be published. All fields are required