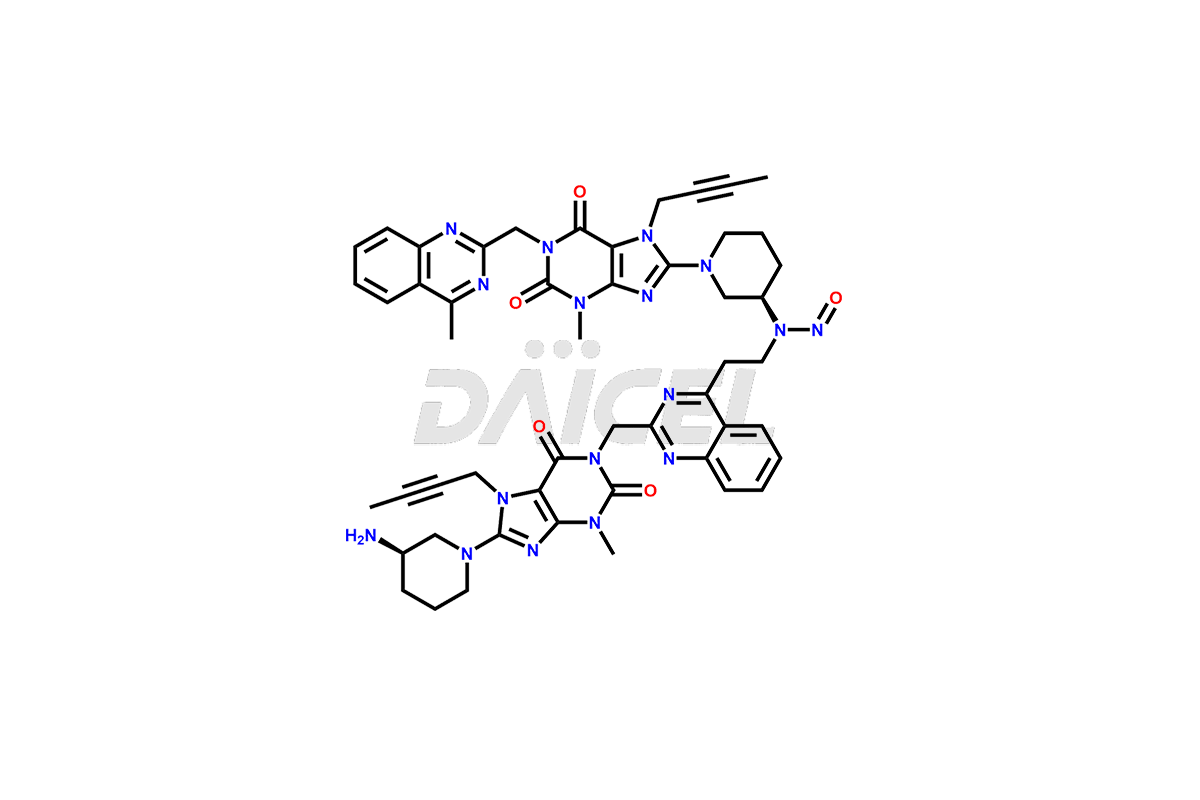
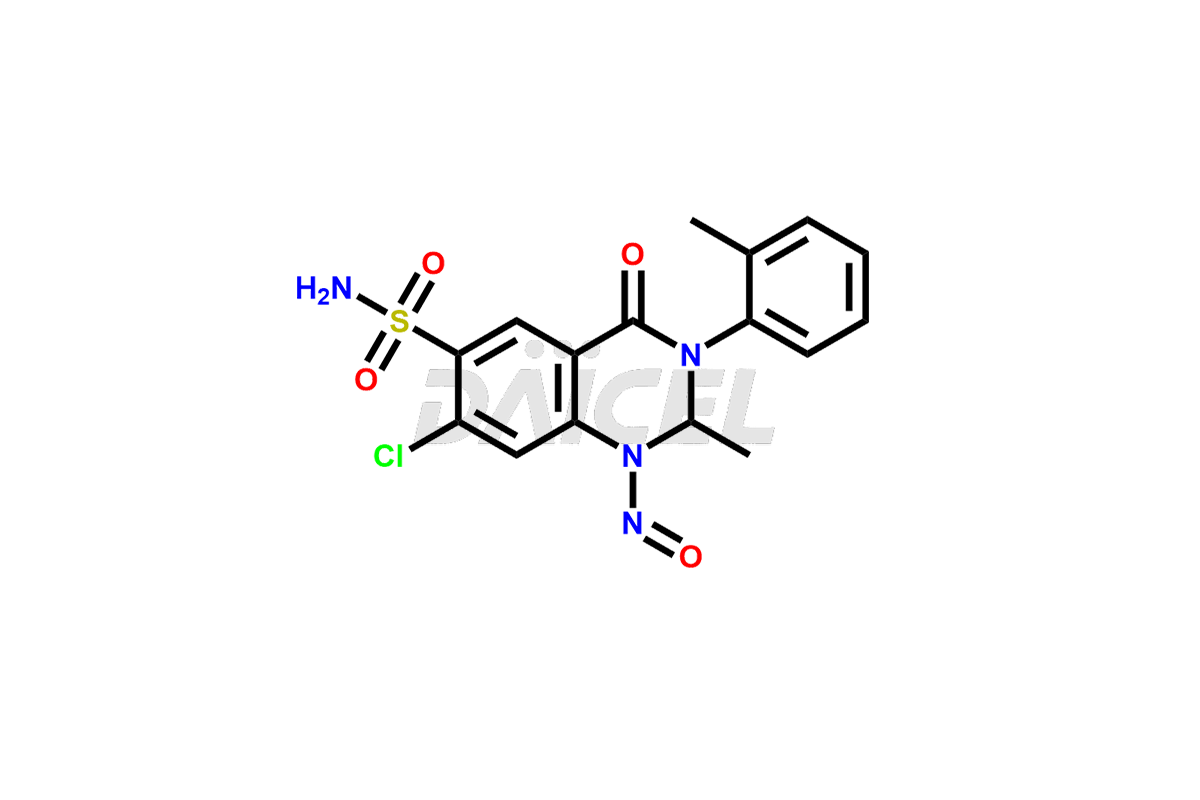
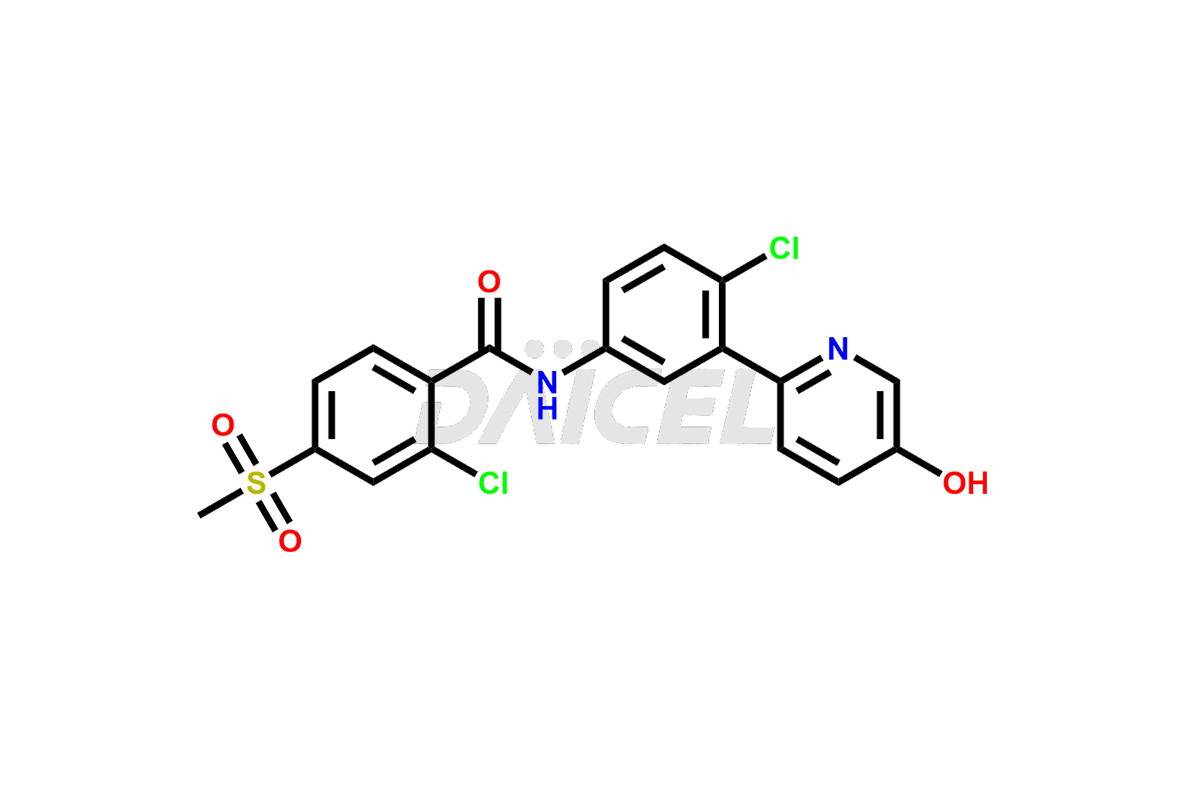
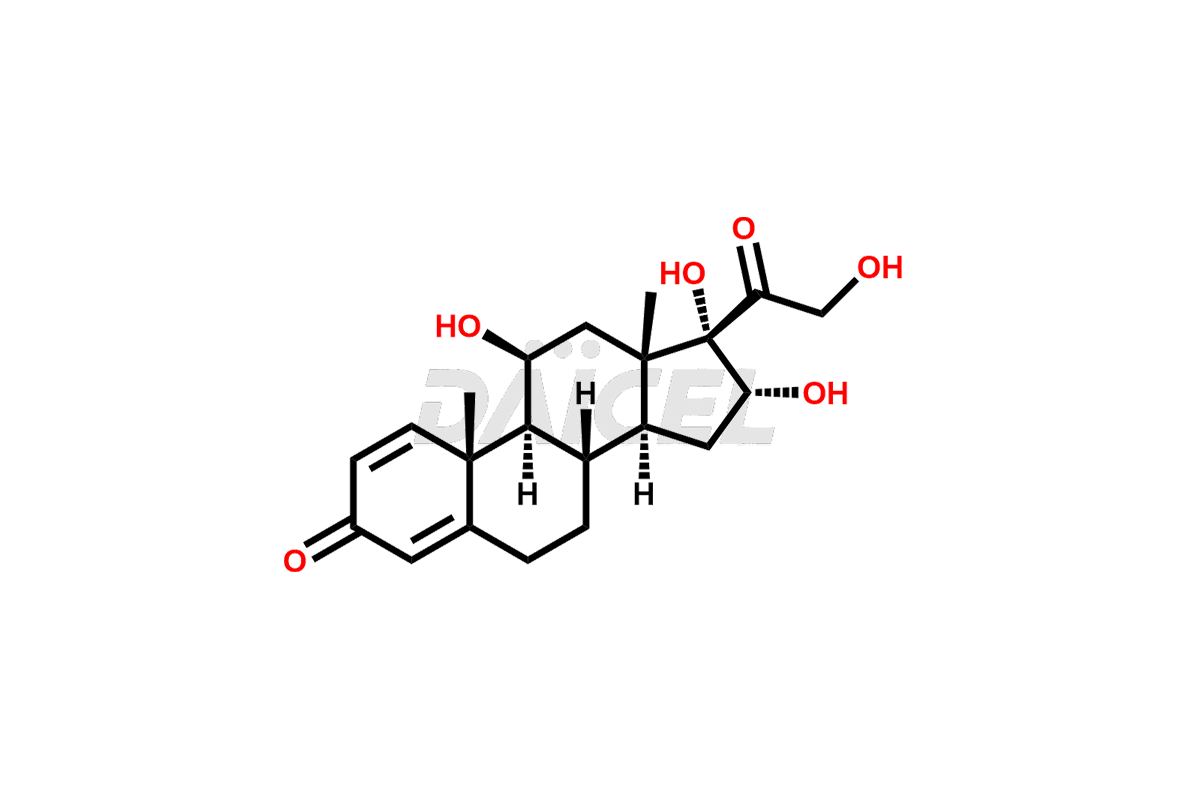
Latest Updates:
Pharma Standards
![]()
Daicel Pharma Standards offers a wide range of high-quality pharmaceutical standards in quantities ranging from milligram to multi gram scale.
Custom Synthesis
![]()
We offer Custom synthesis of Impurities, Metabolites and Stable Isotope labelled API Standards. We offer Custom synthesis of Peptide, Peptide Impurities and Stable Isotope Labelled Peptide Standards API’s
Pharma Services
![]()
We offer broad range of Separation, Purification and Analytical Services to assist customers in their Regulatory filings
Daicel-Sustainable Value Together
As a global leader in drug impurity standards, drug metabolites, and stable isotope-labelled compounds, we drive innovation in pharmaceutical development.
From synthesis to supply, we support every stage of the product development cycle, ensuring access to high-quality impurities and labelled compounds. Leveraging our in-house expertise, we’ve built an extensive catalogue spanning everything from small molecules to complex peptides, tailored to meet diverse research and production needs.