Focus Segment
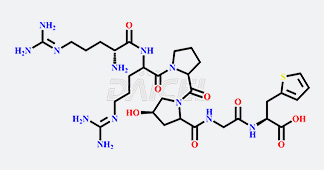
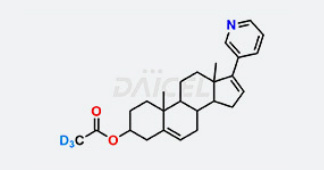
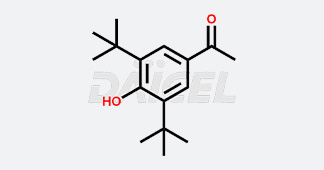
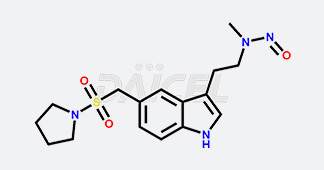
Daicel Chiral Technologies specializes in synthesizing premium quality impurities for Semaglutide, Liraglutide, and Tirzepatide, leveraging our cutting-edge synthesis capabilities and extensive peptide chemistry expertise. These impurity standards play a critical role in evaluating the quality, stability, and efficacy of GLP-1 drugs. To ensure the utmost reliability and compliance, we provide Certificates of Analysis (CoA) from our GMP-compliant analytical facility, addressing the stringent requirements of pharmaceutical research and development.
Order Information
Daicel Products Everywhere
About Us
We support throughout the Product development cycle by synthesizing and supplying Impurities & Labelled compounds.
Daicel Pharma Standards with a diverse combination of skills, resources and capabilities facilitate research, isolation and characterization of unknown impurities thus providing a perfect platform to assist you in meeting the regulatory requirements. We work closely with you to understand your requirements and offer time bound solutions.
FAQ's
GLP impurities are a subset of impurities that are specifically relevant to pharmaceutical products and are regulated under Good Laboratory Practice guidelines. Unlike organic or inorganic impurities, GLP impurities are characterized by their potential impact on the safety or efficacy of pharmaceuticals.
Analytical techniques such as chromatography (HPLC, GC), spectroscopy (UV-Vis, IR), mass spectrometry (MS), and nuclear magnetic resonance (NMR) are commonly employed to detect and quantify.
Manufacturers can employ various strategies, including stringent raw material selection, optimized synthetic routes, purification processes, and strict quality control measures to minimize GLP impurities during manufacturing.
Exceeding permissible limits of GLP impurities in pharmaceutical products can lead to regulatory non-compliance, jeopardizing product approval and market availability. Additionally, high levels of impurities may compromise product safety and efficacy, potentially causing adverse effects in patients