Valganciclovir
General Information
Valganciclovir Impurities and Valganciclovir
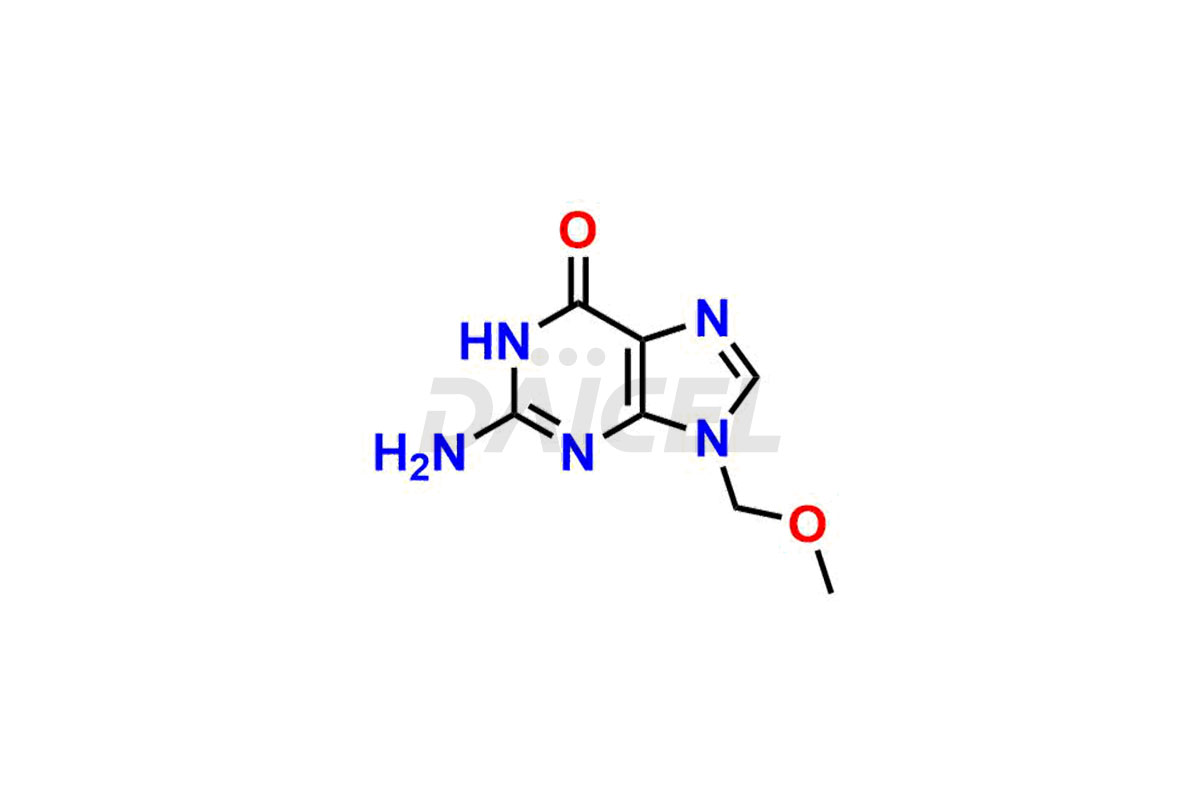
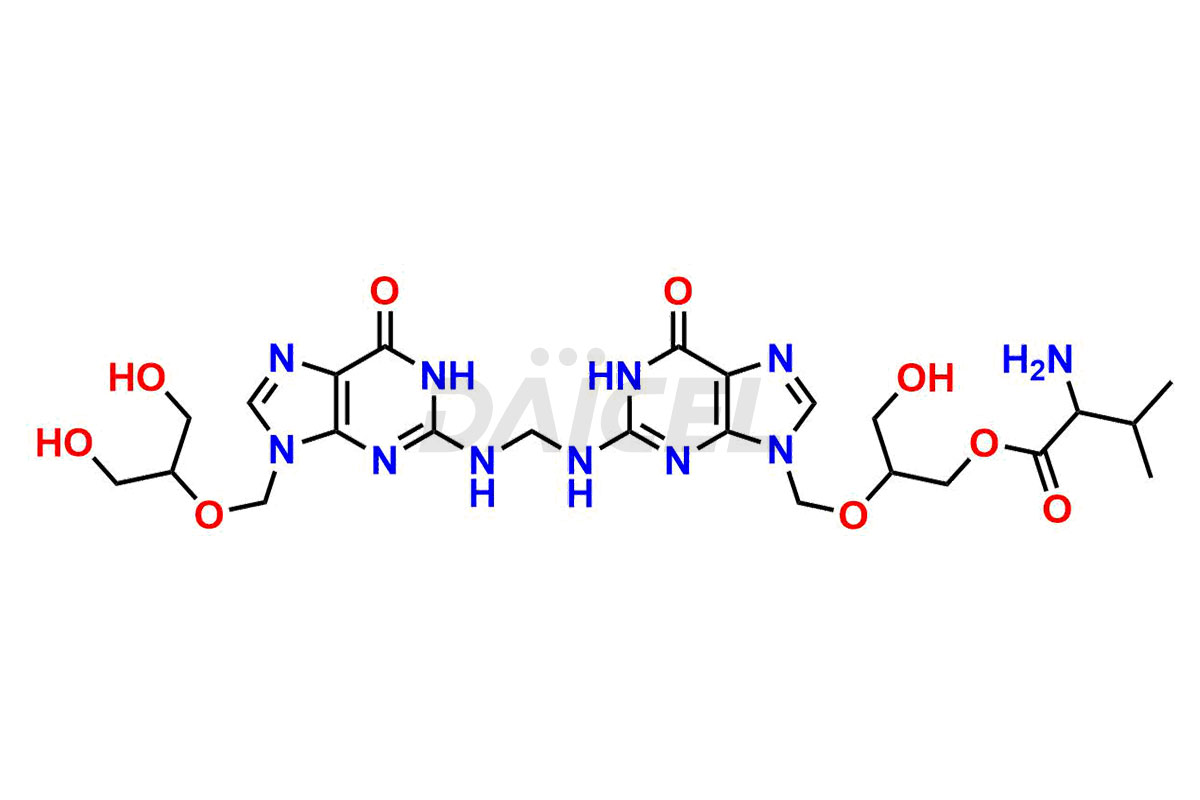
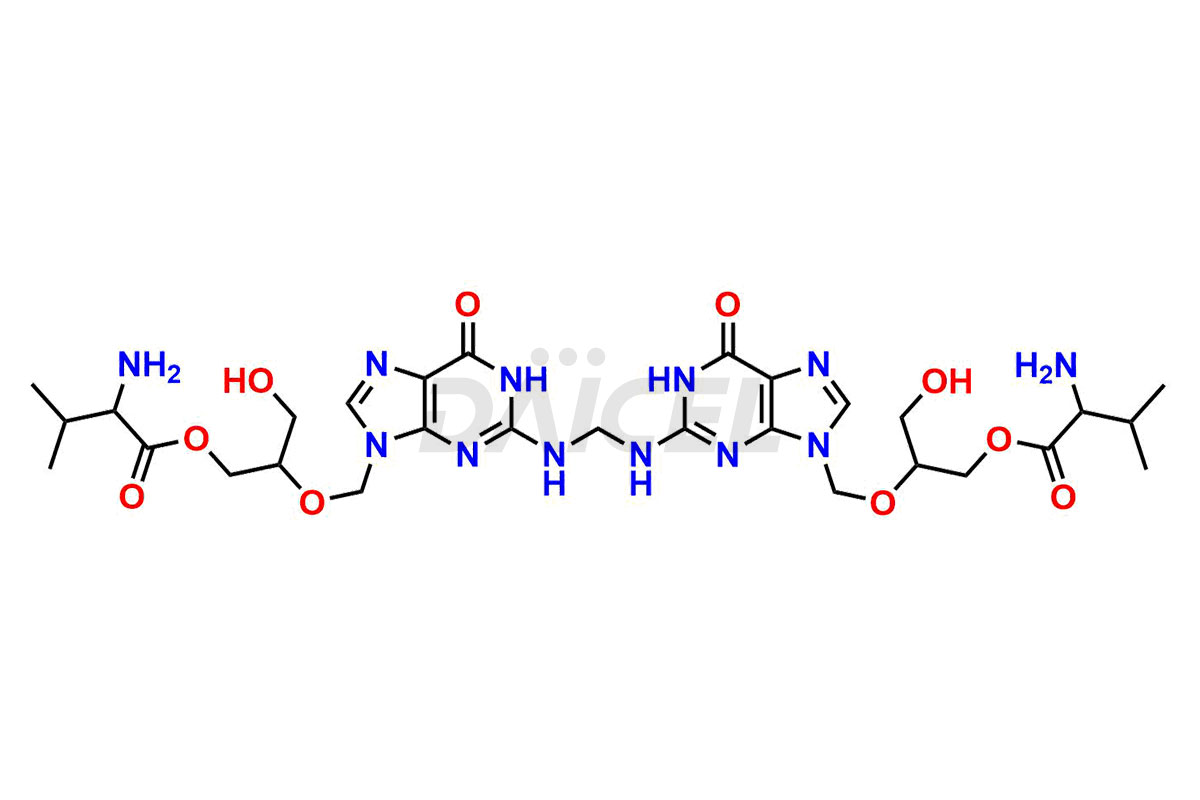
Daicel Pharma is a trusted provider of quality Valganciclovir impurity standards, including 2-amino-9-(methoxymethyl)-1,9-dihydro-6H-purin-6-one, Chloro Diastereoisomer of Valganciclovir, Isovalganciclovir, Mono des valine valganciclovir dimer, Valganciclovir Dimer Impurity (Mixture of stereoisomers A, B & C), and Valganciclovir N-Valyl Impurity. These impurities are crucial in meticulously evaluating the quality, stability, and safety of the active pharmaceutical ingredient, Valganciclovir. Furthermore, Daicel Pharma customizes Valganciclovir impurities, guaranteeing that it meets individual client specifications. With global shipping capabilities, they can be conveniently delivered to customers worldwide, offering unparalleled convenience.
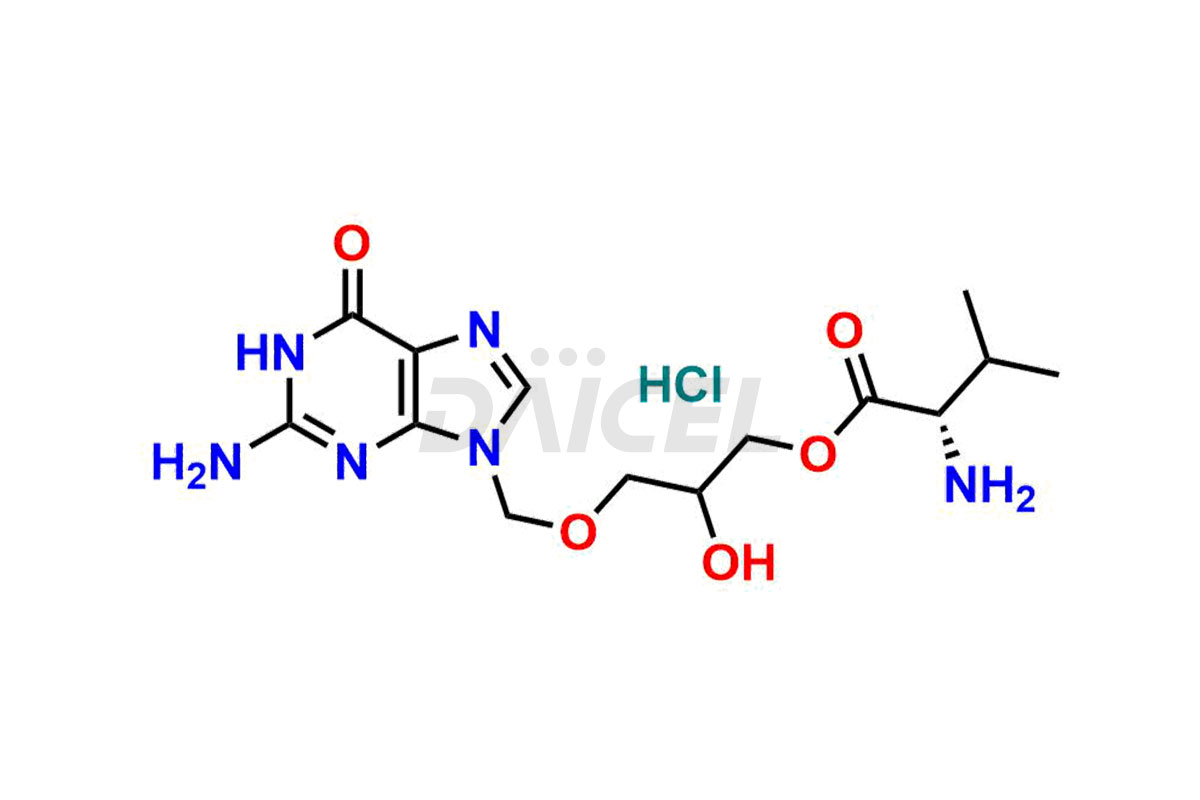
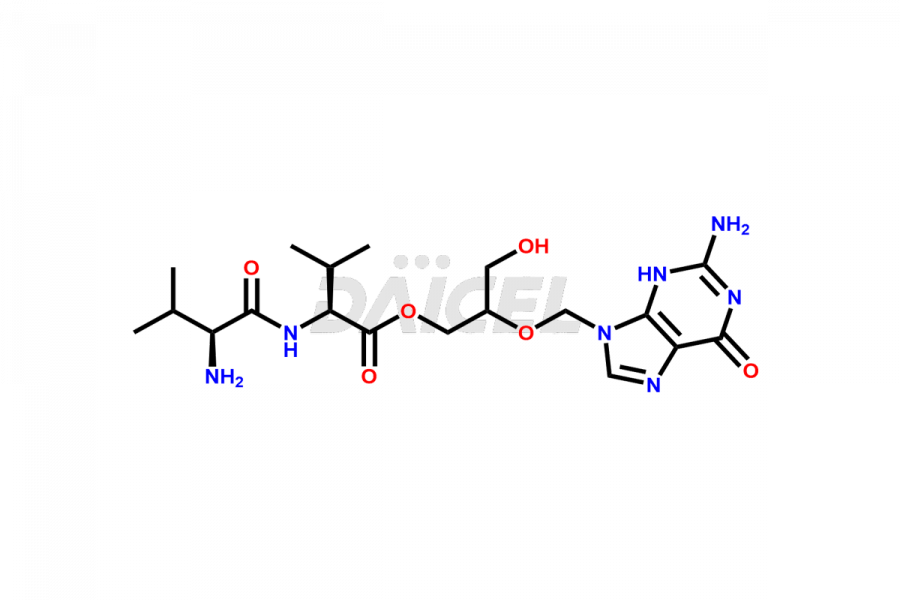
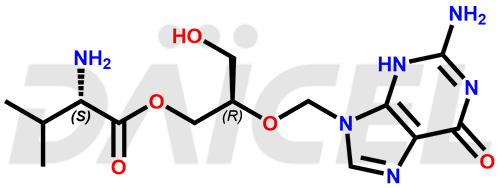
Valganciclovir [CAS: 175865-60-8] is a L-valinyl ester of ganciclovir. It is an antiviral medication that treats cytomegalovirus (CMV) diseases. It effectively manages CMV retinitis in patients with AIDS.
Valganciclovir: Use and Commercial Availability
Valganciclovir is used to treat cytomegalovirus (CMV) retinitis, an eye infection that can occur in individuals with AIDS. It also helps prevent CMV disease in patients who have undergone organ transplantation. Valganciclovir is available under the brand name Valcyte.
Valganciclovir Structure and Mechanism of Action
The chemical name of Valganciclovir is 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]-3-hydroxypropyl ester L-Valine. Its chemical formula is C14H22N6O5, and its molecular weight is approximately 354.36 g/mol.
Valganciclovir is a prodrug of ganciclovir, consisting of a mixture of two diastereomers. Ganciclovir inhibits human cytomegalovirus replication.
Valganciclovir Impurities and Synthesis
Valganciclovir impurities can arise during synthesis1 due to the storage or use of specific raw materials and intermediates in manufacturing. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Valganciclovir for patient use.
Daicel Pharma provides a comprehensive Certificate of Analysis (CoA) for Valganciclovir impurity standards, such as 2-amino-9-(methoxymethyl)-1,9-dihydro-6H-purin-6-one, Chloro Diastereoisomer of Valganciclovir, Isovalganciclovir, Mono des valine valganciclovir dimer, Valganciclovir Dimer Impurity (Mixture of stereoisomers A, B & C), and Valganciclovir N-Valyl Impurity. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, upon delivery, 13C-DEPT is also provided. Daicel possesses the technology and expertise to synthesize any unknown Valganciclovir impurity or degradation product.
References
FAQ's
References
- Nestor, John J.; Womble, Scott W.; Maag, Hans, 2-(2-amino-1,6-dihydro-6-oxo-purin-9-yl)methoxy-1,3-propanediol derivatives, F. Hoffmann-La Roche Ag, Switzerland, EP694547B1, April 21, 1999 ()
- Xu, Hong-Rong; Li, Xue-Ning; Chen, Wei-Li; Liu, Gang-Yi; Chu, Nan-Nan; Yu, Chen, A sensitive assay for simultaneous determination of plasma concentrations of valganciclovir and its active metabolite ganciclovir by LC/MS/MS, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 848, Issue: 2, Pages: 329-334, 2007
Frequently Asked Questions
Are Valganciclovir impurities tested for potential toxicity?
Yes, impurities in Valganciclovir undergo toxicity testing to assess their potentially harmful effects. The tests help determine their safety profile and establish acceptable limits to ensure patient safety.
Which solvent helps in analyzing Valganciclovir impurities?
Dimethyl sulfoxide (DMSO) and Acetonitrile are the solvents for analyzing many Valganciclovir impurities.
Which are the analytical methods used to analyze Valganciclovir impurities?
Reverse Phase-High-performance liquid chromatography (RP-HPLC) is employed to separate and analyze the impurities of Valganciclovir.
How should Valganciclovir impurities be stored in terms of temperature?
The recommendation is to store Valganciclovir impurities at room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.