LOAD MORE
You're viewed 9 of 10 products
Daicel Pharma specializes in synthesizing high-quality Tolvaptan impurities, such as 2-Desmethylbenzaldehyde Tolvaptan, 2-methyl-4-(2-methylbenzamido)benzoic acid, 3-Methyl tolvaptan, 7-chloro-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-ol, Tolvaptan amide impurity, Tolvaptan Open Ring Hydroxyacid, and more. These impurities analyze the active pharmaceutical ingredient of Tolvaptan, ensuring its integrity, stability, and biological safety. Additionally, Daicel Pharma offers tailored synthesis services for Tolvaptan impurities to meet customers’ specific requirements worldwide and trust for exceptional pharmaceutical solutions.
Tolvaptan [CAS: 150683-30-0] is a vasopressin2 receptor antagonist for slowing cyst development progression and renal insufficiency of autosomal dominant polycystic kidney disease (ADPKD) in adults.
Tolvaptan slows kidney function failure in adult patients with progressing autosomal dominant polycystic kidney disease (ADPKD). It also treats hyponatremia secondary to the syndrome of inappropriate antidiuretic-hormone secretion (SIADH).
Tolvaptan is available under Jynarque and Samsca, which contain the active ingredient Tolvaptan.
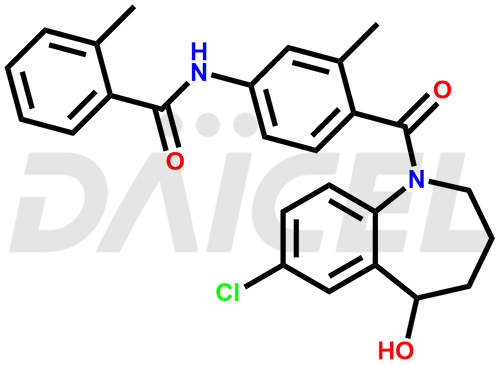
The chemical name of Tolvaptan is N-[4-[(7-Chloro-2,3,4,5-tetrahydro-5-hydroxy-1H-1-benzazepin-1-yl)carbonyl]-3-methylphenyl]-2-methylbenzamide. Its chemical formula is C26H25ClN2O3, and its molecular weight is approximately 448.9 g/mol.
Tolvaptan inhibits native arginine vasopressin (AVP) stimulated cyst growth in human ADPKD epithelial cells.
Effective management and monitoring of impurities are crucial during the manufacturing1 of Tolvaptan to maintain its desired efficacy. These impurities can emerge from various sources, including raw materials, intermediates, and chemicals used in the synthesis. Ensuring the optimal quality and safety of the drug requires dynamic control and oversight of these impurities.
Daicel offers a wholesome and integrated Certificate of Analysis (CoA) for Tolvaptan impurities, such as 2-Desmethylbenzaldehyde Tolvaptan, 2-methyl-4-(2-methylbenzamido)benzoic acid, 3-Methyl tolvaptan, 7-chloro-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-ol, Tolvaptan amide impurity, Tolvaptan Open Ring Hydroxyacid, and more. The Certificate of Analysis (CoA) furnishes comprehensive characterization data, encompassing 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Furthermore, we give a detailed 13C-DEPT analysis upon product delivery. Leveraging advanced technology and expertise, Daicel Pharma can synthesize any unknown Tolvaptan impurity or degradation product.
Many validated analytical methods, such as Reversed Phase high-performance liquid chromatography (RP-HPLC) coupled with a UV method, help detect and quantify impurities in Tolvaptan.
The proper storage conditions, such as temperature control and protection from light and moisture, help to minimize impurity formation.
Methanol helps in analyzing many impurities in Tolvaptan.
Tolvaptan impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.