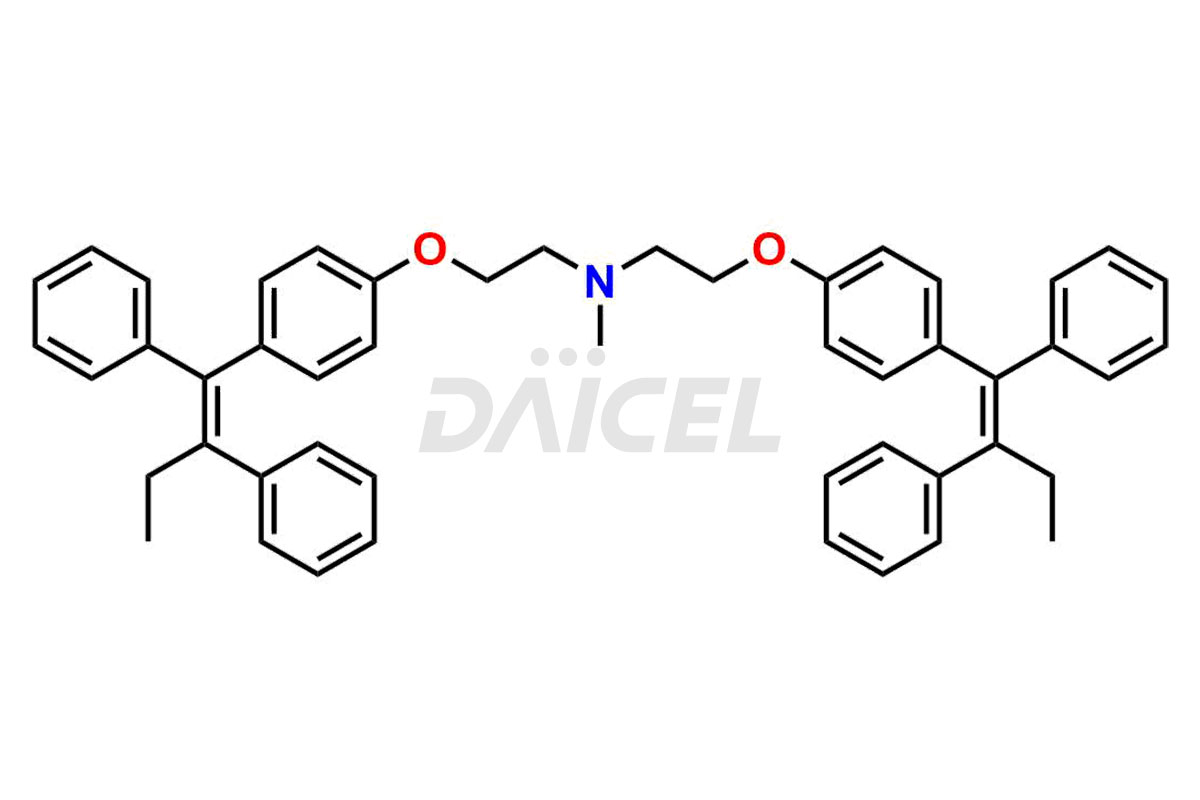
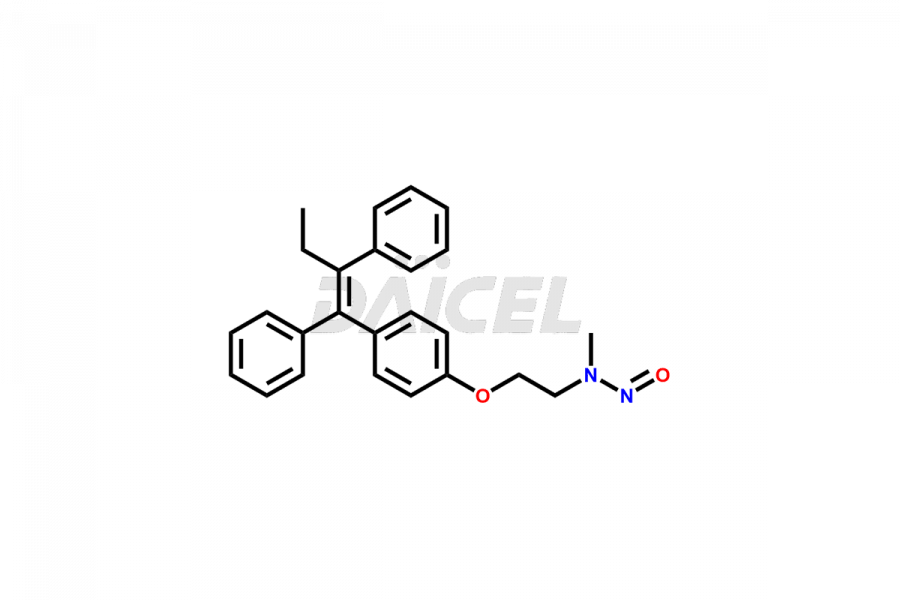
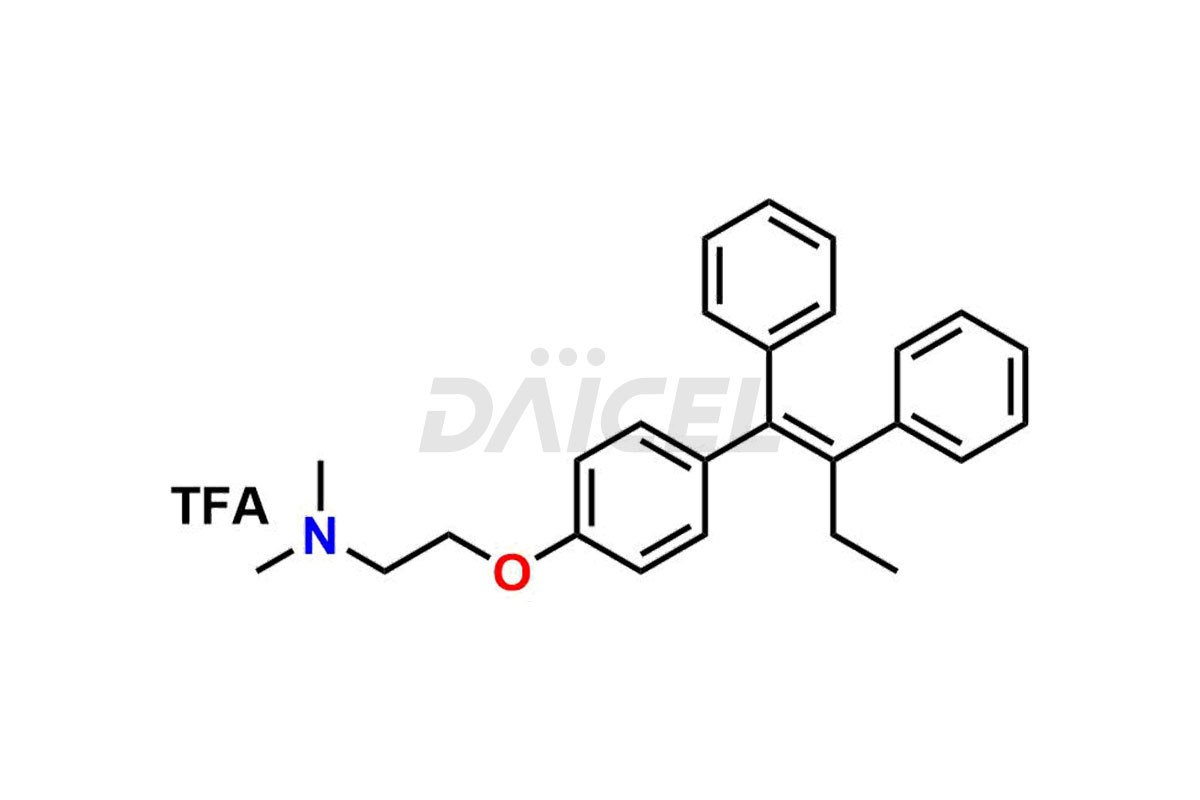
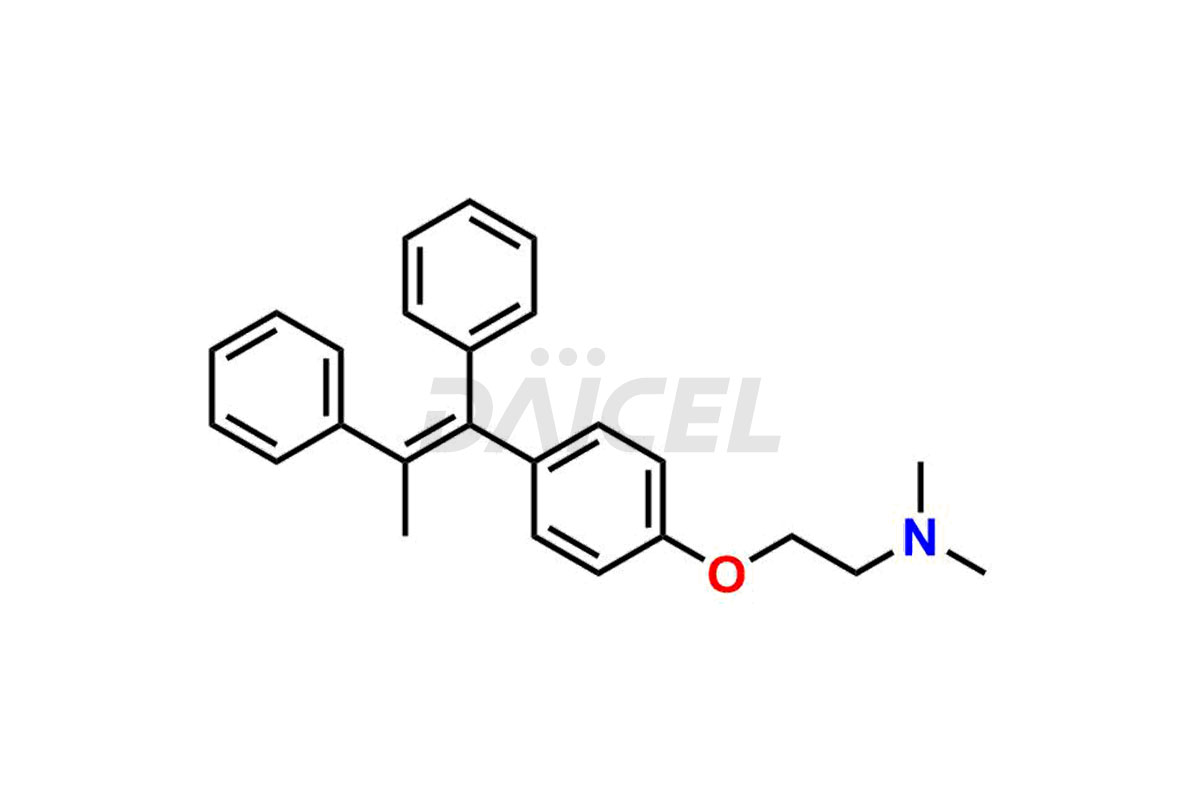
Tamoxifen
General Information
Tamoxifen Impurities and Tamoxifen
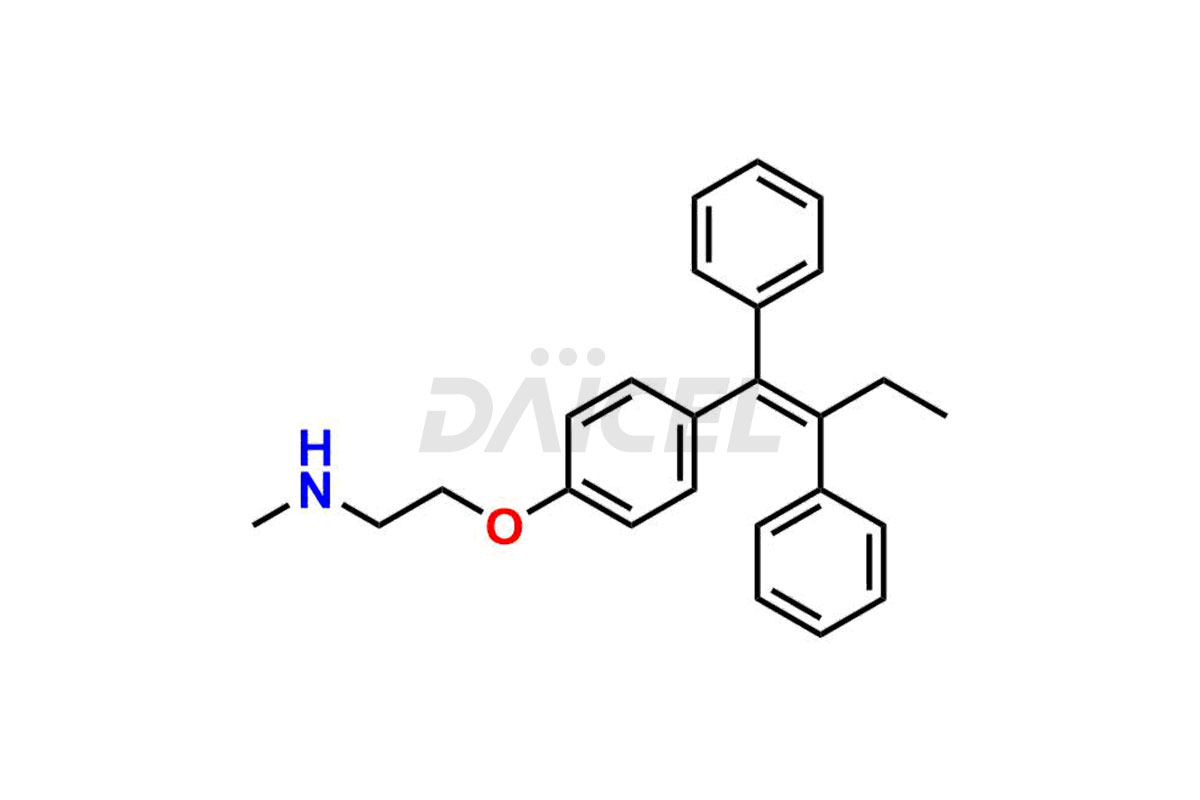
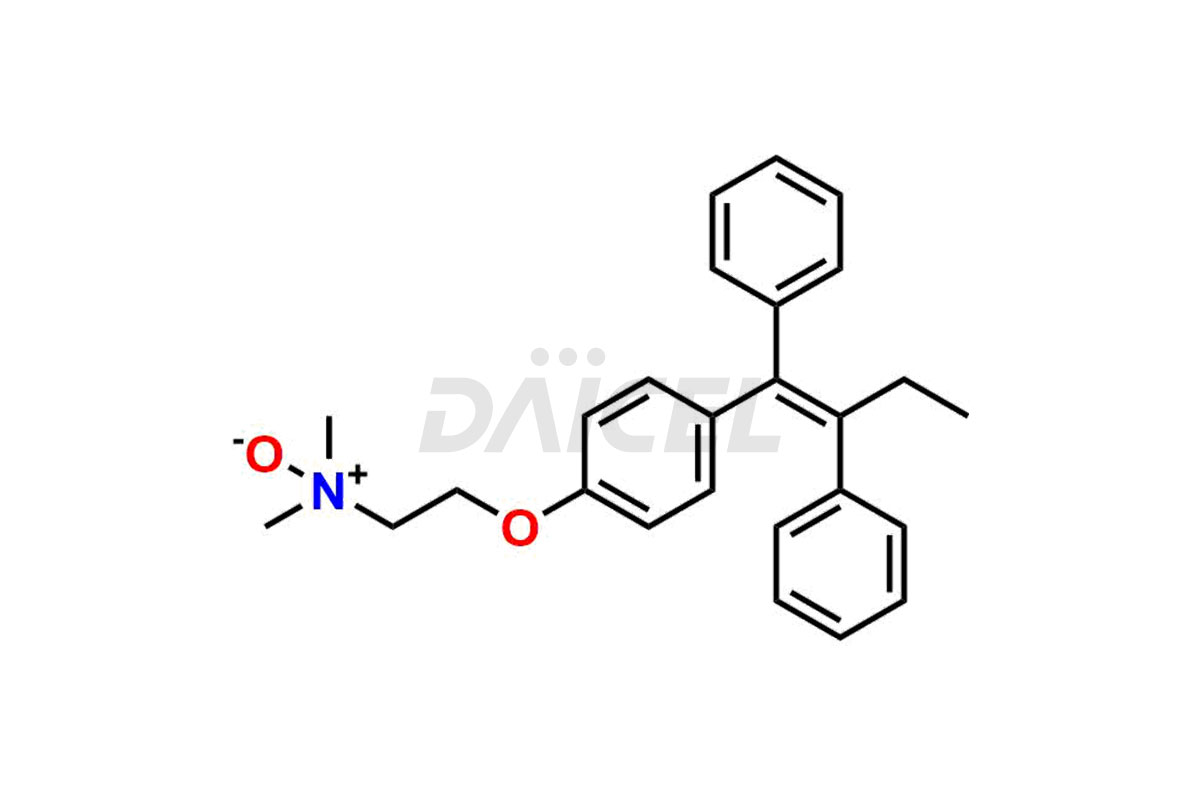
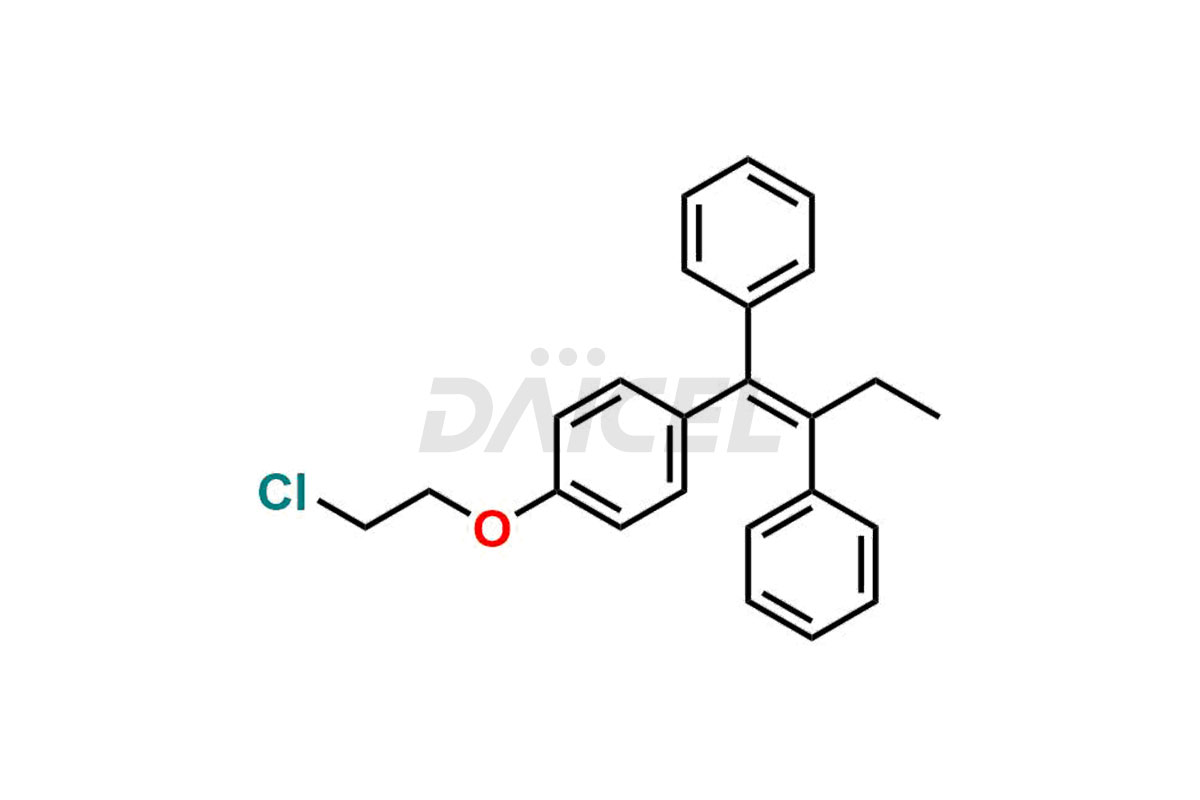
Daicel Pharma provides a wide selection of unique Tamoxifen impurities, including Bis-tamoxifen, Tamoxifen Impurity A, Tamoxifen Impurity D, Tamoxifen Impurity F, Tamoxifen N-Oxide, and Z-Chlorolefin. These impurities are vital in evaluating the active pharmaceutical ingredient Tamoxifen’s quality, stability, and biological safety. Moreover, Daicel Pharma can synthesize Tamoxifen impurities based on specific customer requirements, ensuring reliable delivery worldwide.
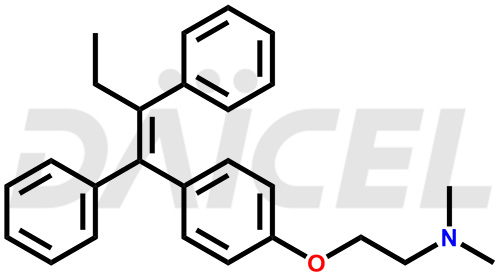
Tamoxifen [CAS: 10540-29-1] is a triphenylethylene derivative that treats breast cancer in adults with estrogen receptor-positive tumors. It is also an adjuvant therapy for early-stage estrogen receptor-positive breast cancer in adults.
Tamoxifen: Use and Commercial Availability
Tamoxifen, a non-steroidal weak estrogen, treats breast cancer at all stages in specific patients. It is for the palliative treatment of premenopausal women with estrogen receptor-positive (ER-positive) disease. Tamoxifen also lowers the risk of invasive breast cancer following surgery and radiation in adult women diagnosed with ductal carcinoma in situ.
Tamoxifen is available under Nolvadex and Soltamox, which contain the active ingredient, Tamoxifen.
Tamoxifen Structure and Mechanism of Action 
The chemical name of Tamoxifen is 2-[4-[(1Z)-1,2-Diphenyl-1-buten-1-yl]phenoxy]-N,N-dimethylethanamine. Its chemical formula is C26H29NO, and its molecular weight is approximately 371.5 g/mol.
The anti-estrogenic effects of Tamoxifen compete with estrogen for the binding sites in breast tissues.
Tamoxifen Impurities and Synthesis
During the synthesis1 and storage of Tamoxifen, various impurities such as related substances, degradation products, and residual solvents can form. It is crucial to diligently monitor and control these impurities to ensure the medication’s safety, effectiveness, and overall quality.
Daicel Pharma provides a comprehensive Certificate of Analysis (CoA) for Tamoxifen impurity standards, including Bis-tamoxifen, Tamoxifen Impurity A, Tamoxifen Impurity D, Tamoxifen Impurity F, Tamoxifen N-Oxide, and Z-Chlorolefin. These impurities are produced strictly with current Good Manufacturing Practices (cGMP). The CoA comprises detailed characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity, offering a thorough understanding of the impurity profile. Upon request, Daicel can also supply 13C-DEPT data for further characterization.
Additionally, Daicel Pharma possesses the technical expertise to synthesize any unknown impurities or degradation products of Tamoxifen.
References
FAQ's
References
- Brittain, David Robert, Process for The Manufacture Of 1,1,2-Triphenylalk-1-Enes, Imperial Chemical Industries Ltd., GB1354939A, May 30, 1974
- Daniel, C. P.; Gaskell, S. J.; Bishop, G.; Nicholson, R. I., Determination of tamoxifen and an hydroxylated metabolite in plasma from patients with advanced breast cancer using gas chromatography-mass spectrometry, Journal of Endocrinology, Volume: 83, Issue: 3, Pages: 401-8, 1979
Frequently Asked Questions
How are Tamoxifen impurities detected and quantified?
Analytical Methods such as High-Performance Liquid Chromatography (HPLC) can detect impurities in Tamoxifen.
Can Tamoxifen impurities affect patient safety?
The presence of impurities in Tamoxifen can affect patient safety. The type and level of these impurities can lead to adverse effects or diminish the effectiveness of the medication.
Which solvents help in the analysis of Tamoxifen impurities?
Methanol is used to achieve optimal solubility and separation of Tamoxifen impurities.
What are the temperature conditions required to store Tamoxifen impurities?
Tamoxifen impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.