Siponimod
General Information
Siponimod Impurities and Siponimod
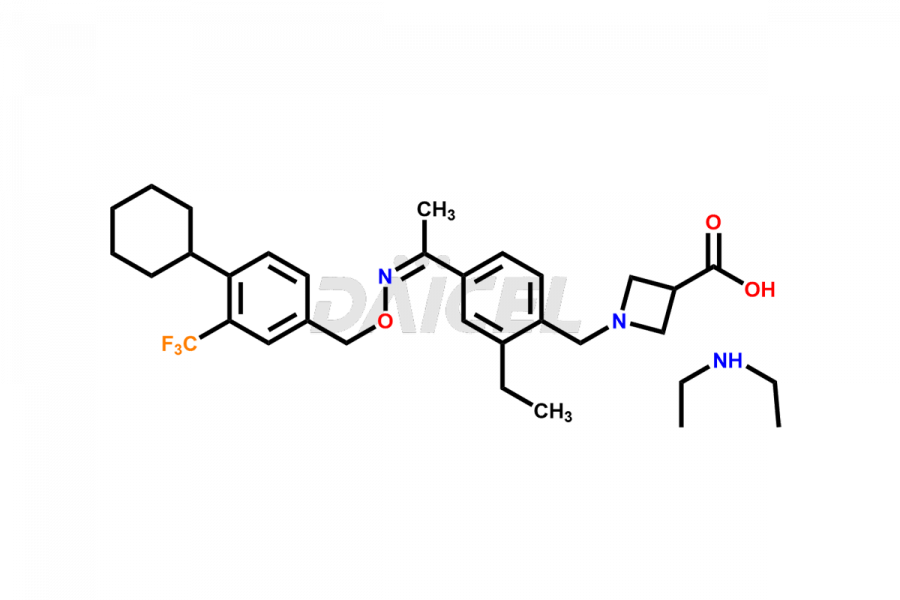
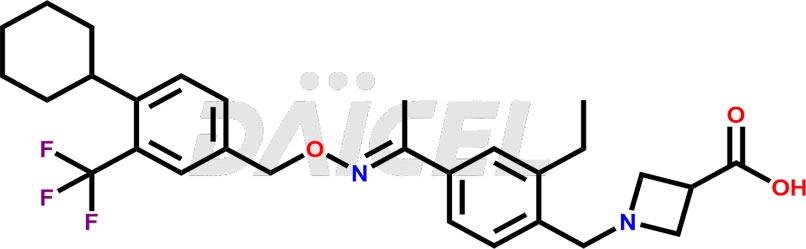
Daicel Pharma is a reliable partner for synthesizing high-quality Siponimod impurities, specifically 1-(4-ethyl-3-(hydroxymethyl)phenyl)ethan-1-one, Siponimod M17, and Siponimod Z-Isomer diethylamine salt. These impurities help assess the quality, stability, and safety of the active pharmaceutical ingredient, Siponimod. Daicel Pharma also offers custom synthesis of Siponimod impurities, which can be shipped worldwide to meet customers’ unique requirements.
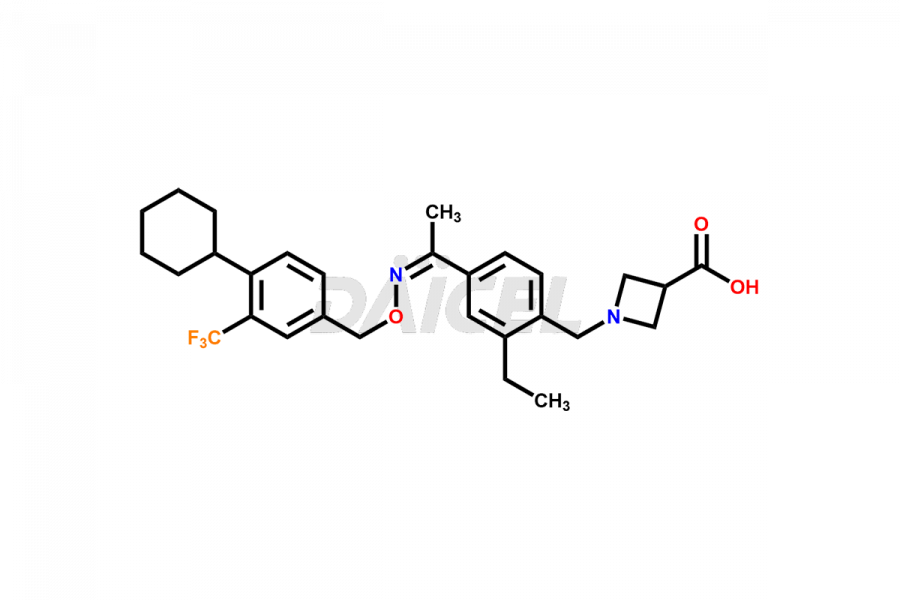
Siponimod [CAS: 1230487-00-9] is a medication that modulates a type of receptor called sphingosine 1-phosphate (S1P). It treats relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.
Siponimod: Use and Commercial Availability
Siponimod is available as Mayzent, an oral medication to treat relapsing forms of multiple sclerosis (MS) in adults. It is a newly approved drug by the US FDA and Health Canada. Siponimod is an immunomodulatory agent treating patients with multiple sclerosis.
Siponimod Structure and Mechanism of Action 
The chemical name of Siponimod is 1-[[4-[(1E)-1-[[[4-Cyclohexyl-3-(trifluoromethyl)phenyl]methoxy]imino]ethyl]-2-ethylphenyl]methyl]-3-azetidinecarboxylic acid. Its chemical formula is C29H35F3N2O3, and its molecular weight is approximately 516.6 g/mol.
Siponimod binds with sphingosine-1-phosphate (SIP) receptors 1 and 5 with affinity. It reduces lymphocyte migration into the central nervous system.
Siponimod Impurities and Synthesis
The impurities in Siponimod form during various stages of the manufacturing process, including synthesis1, purification, and storage. It is essential to identify and control impurities to ensure the safety and efficacy of the final product.
Daicel Pharma issues a Certificate of Analysis (CoA) for Siponimod impurity standards, including 1-(4-ethyl-3-(hydroxymethyl)phenyl)ethan-1-one, Siponimod M17, and Siponimod Z-Isomer diethylamine salt. The CoA is from an analytical facility that complies with current Good Manufacturing Practices (cGMP) and includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization data such as 13C-DEPT and CHN on request. Daicel Pharma can prepare unknown Siponimod impurities or degradation products. A complete characterization report accompanies each delivery.
References
FAQ's
References
- Shifeng Pan, Wenqi Gao, Nathanael S Gray, Yuan Mi, Yi Fan, Immunosuppresant compounds and compositions, Novartis AG, US7939519B2, May 10, 2011
- Li, Wenkui; Luo, Suyi; Smith, Harold T.; Tse, Francis L. S., Quantitative determination of BAF312, a S1P-R modulator, in human urine by LC-MS/MS: Prevention and recovery of lost analyte due to container surface adsorption, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 878, Issue: 5-6, Pages: 583-589, 2010
Frequently Asked Questions
How is the purity of Siponimod assessed?
The purity of Siponimod is assessed using analytical methods such as HPLC or LC-MS to quantify the impurities present. The percentage of the active ingredient present in the drug substance helps calculate its purity.
What is forced degradation in the context of Siponimod?
Forced degradation is a stress testing process used to simulate the effect of different environmental factors on the stability of Siponimod. This process helps identify potential impurities that may form during storage or transportation.
How does Siponimod impurities profiling contribute to the development of Siponimod?
Impurity profiling helps identify and quantify impurities in Siponimod, which can help to optimize the manufacturing process and ensure the quality and safety of the drug.
What are the temperature conditions required to store Siponimod impurities?
Siponimod impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.