Praziquantel
General Information
Praziquantel Impurities and Praziquantel
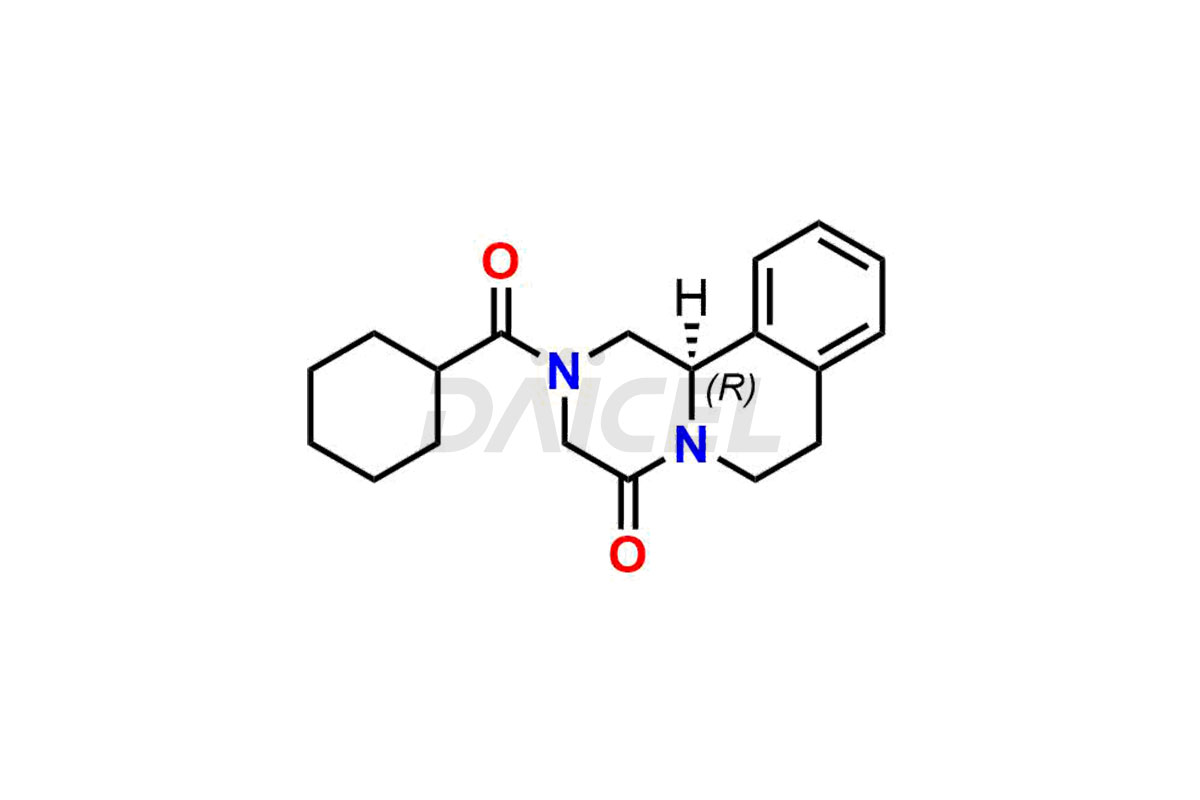
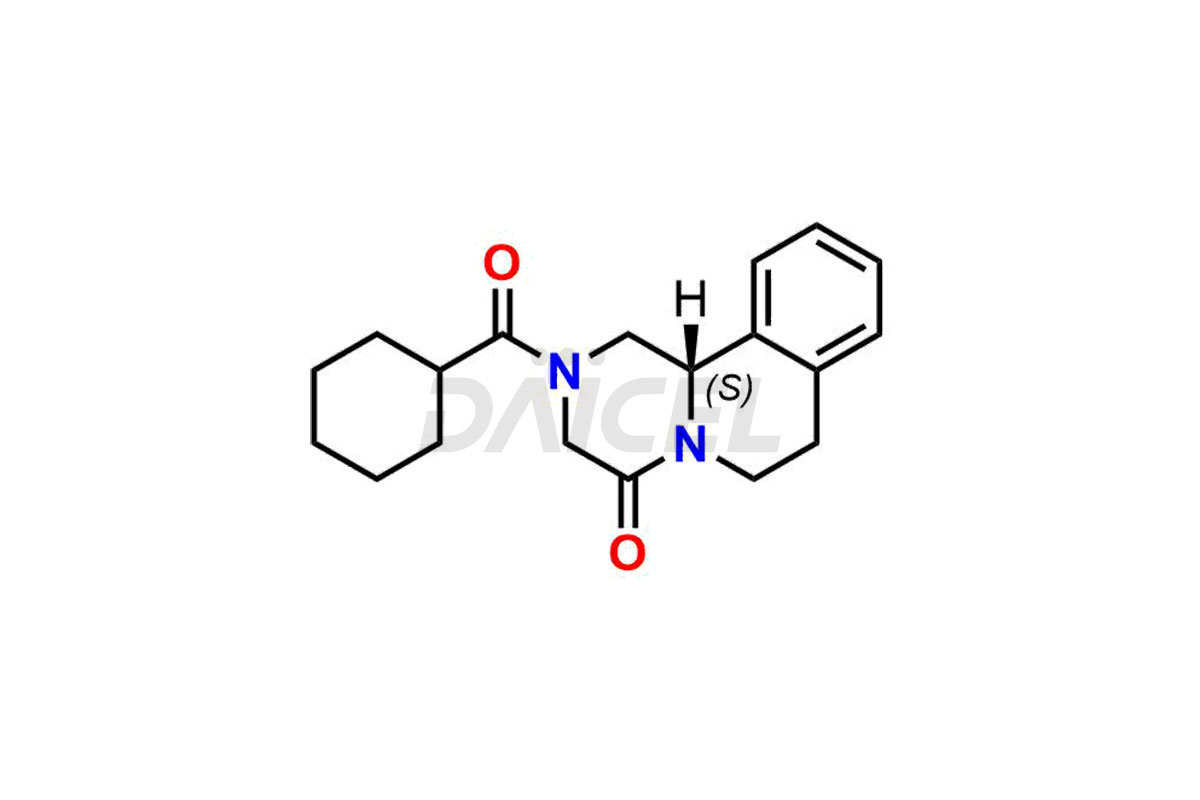
Daicel Pharma synthesizes Praziquantel impurity standards, including S-Praziquantel and R-Praziquantel. Their presence can affect the effectiveness, stability, and safety of Praziquantel. Also, Daicel Pharma synthesizes custom Praziquantel impurities and delivers them globally.
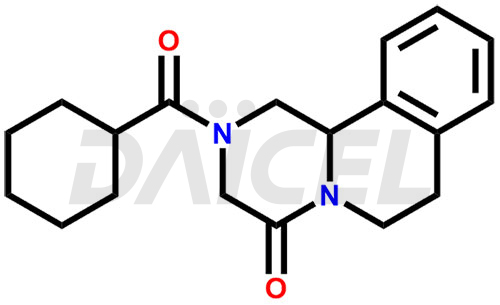
Praziquantel [CAS: 55268-74-1] is a medication that acts as an anthelmintic agent, effectively targeting a wide range of trematodes and cestodes. It treats schistosomiasis, liver flukes, and cysticercosis.
Praziquantel: Use and Commercial Availability
Praziquantel is a versatile medication that effectively treats infections caused by various parasites. It is a broad-spectrum vermicide widely used to control cestodes (tapeworms) such as Taenia saginata, Diphyllobothrium latum, and Taenia solium. Moreover, Praziquantel is also highly effective in managing infections caused by the parasite Hymenolepis nana. Furthermore, it is the preferred drug for treating infections caused by trematodes (flukes) like Schistosoma sp., Clonorchis sinensis, and Paragonimus westermani. This drug is available under the tradename of Biltricide.
Praziquantel Structure and Mechanism of Action
The chemical name of Praziquantel is 2-(Cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino[2,1-a]isoquinolin-4-one. Its chemical formula is C19H24N2O2, and its molecular weight is approximately 312.4 g/mol.
Praziquantel causes a rapid contraction of schistosomes due to the permeability of the cell membrane.
Praziquantel Impurities and Synthesis
Praziquantel impurities can arise as byproducts or be introduced during the synthetic process1, due to reaction conditions, starting materials, and purification methods. These impurities, if present, can affect the drug’s overall quality, efficacy, and safety.
Daicel Pharma provides a Certificate of Analysis (CoA) for Praziquantel impurity standards such as S-Praziquantel and R-Praziquantel. Our cGMP-certified analytical facility gives a comprehensive CoA with detailed characterization data like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterizations like 13C-DEPT are available upon request. At Daicel Pharma, our team of experts synthesizes Praziquantel impurities.
References
FAQ's
References
- Seubert, Juergen; Thomas, Herbert; Andrews, Peter, Pyrazino-Isoquinoline Derivatives, Merck Patent G.m.b.H., United States, GB1441554A, July 7, 1976
- Diekmann, H. W.,Quantitative determination of praziquantel in body fluids by gas liquid chromatography, European Journal of Drug Metabolism and Pharmacokinetics, Volume: 4, Issue: 3, Pages: 139-41,1979
Frequently Asked Questions
How are Praziquantel impurities managed during manufacturing?
Planning and optimization of Praziquantel's synthetic method minimizes impurity formation. Reaction parameters of temperature, pressure, and reaction time are under strict control to minimize impurity production. Side reactions and impurity production reduce by optimizing reaction conditions. Throughout the production process, stringent quality control mechanisms are in place.
Can Praziquantel impurities change over time?
Yes, the impurities in Praziquantel can alter over time. A pharmaceutical product's stability, including its formation and degradation, can be impacted by storage conditions, temperature, humidity, light exposure, and the chemical characteristics of the therapeutic ingredient.
Which solvent is used for the analysis of Praziquantel impurities?
The solvent used to analyze Praziquantel impurities might vary based on the studies of the individual impurity and its analytical method.
What are the temperature conditions required to store Praziquantel impurities?
Praziquantel impurities should be stored at a controlled room temperature of 2-8°C or as described on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.