Plecanatide
General Information
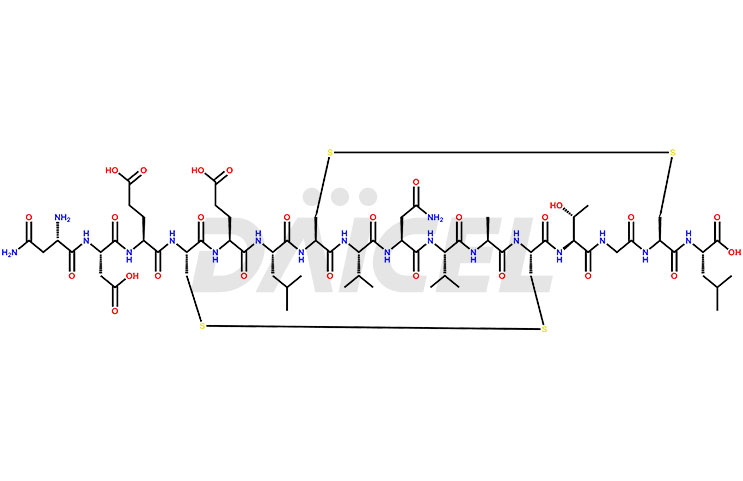
Plecanatide Impurities and Plecanatide
Daicel Pharma synthesizes high-quality Plecanatide impurities like Des-Asn(1)-Plecanatide, that helps in the quality, stability, and biological safety analysis of Plecanatide. We also offer custom synthesis of Plecanatide impurities and supply worldwide.
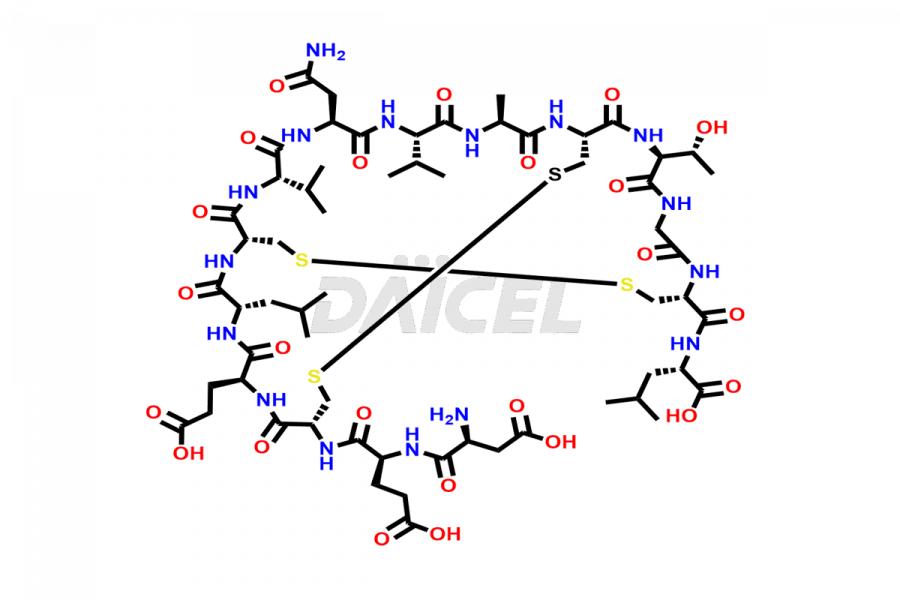
Plecanatide [CAS: 467426-54-6] is a 16 amino acid peptide, a laxative, used to treat chronic idiopathic constipation and irritable bowel syndrome (IBS) with constipation.
Plecanatide : Use and Commercial Availability
Plecanatide belongs to a group of drugs known as guanylate cyclase-C agonists that increase the gastrointestinal tract’s movement by boosting fluid secretion. This medication is primarily used in adults to alleviate chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C), conditions characterized by infrequent bowel movements, abdominal discomfort, bloating, and difficulty passing stools that are not due to underlying diseases or medication. Plecanatide is available as an oral tablet for use by adults under the brand name Trulance.
Plecanatide Structure and Mechanism of Action
The chemical formula of Plecanatide is C65H104N18O26S4, and its molecular weight is approximately 1681.89 g/mol.
Plecanatide functions as a guanylate cyclase C (GC-C) agonist and is structurally related to human uroguanylin. Plecanatide and its active metabolite bind to GC-C and act locally on the luminal surface of the intestinal epithelium. The activation of GC-C causes an increase in the intracellular and extracellular concentrations of cyclic guanosine monophosphate (cGMP), which stimulates the secretion of chloride and bicarbonate into the intestinal lumen. It causes increased intestinal fluid and accelerated transit.
Plecanatide Impurities and Synthesis
Organic or inorganic impurities may form during the manufacturing process1 of Plecanatide or as a result of the degradation of the drug substance or drug product over time. To ensure the safety and efficacy of Plecanatide, regulatory authorities require pharmaceutical companies to perform extensive analytical testing to identify and quantify impurities in the drug substance and drug product.
At Daicel, we provide a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Plecanatide impurity standard, Des-Asn(1)-Plecanatide, with complete characterization data including 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Upon request, we provide 13C-DEPT and CHN. Also, we provide a complete characterization report upon delivery. Daicel offers highly pure isotope-labeled standards of Plecanatide in bioanalytical research and BA/BE studies with isotope data in CoA.
References
- Kunwar Shailubhai, Gregory Nikiforovich; Gary S. Jacob; Callisto Pharmaceuticals, US, “Guanylate Cyclase Receptor Agonists For The Treatment Of Tissue Inflammation And Carcinogenesis”, US patent, US 7,041,786 B2, May 9, 2006
- Shailubhai, Kunwar; Comiskey, Stephen; Feng, Rong; Bai, Juncai; Zhang, Ruoping; Jia, Jun; Zhou, Junfeng; Zhao, Qiao; Zhang, Guoqing; Synergy Pharmaceuticals, Inc., “Preparation of ultra-pure guanylate cyclase C agonist peptides by purification via solvent exchange-lyophilization” US patent, US 10,011,637 Β2, July 3, 2018
Frequently Asked Questions
Why is the synthesis of Plecanatide impurities important?
Plecanatide impurities can affect the safety and efficacy of the medication. So, regulatory agencies such as the FDA require manufacturers to identify and quantify Plecanatide impurities in their products. Hence, pure forms of Plecanatide impurities are essential for manufacturers to develop better processes.
What are the methods used for synthesizing Plecanatide impurities?
There are many methods for synthesizing Plecanatide impurities, including chemical, enzymatic, and microbial synthesis.
How are Plecanatide impurities identified and quantified?
Plecanatide impurities are identified and quantified using a combination of analytical techniques, such as high-performance liquid chromatography (HPLC), and liquid chromatography-mass spectrometry (LC-MS). These techniques can identify the specific impurities present in the medication and determine their concentration.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.