IOHEXOL
General Information
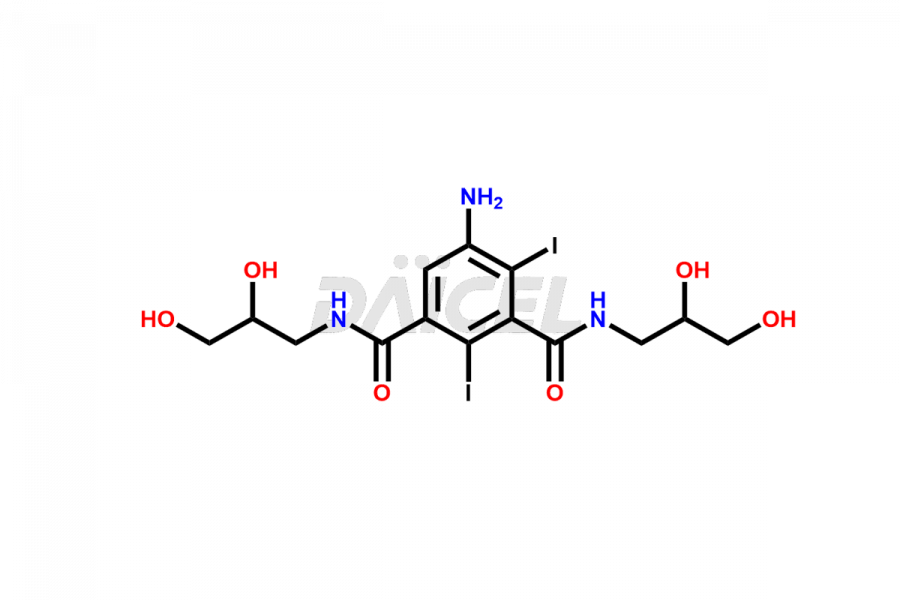
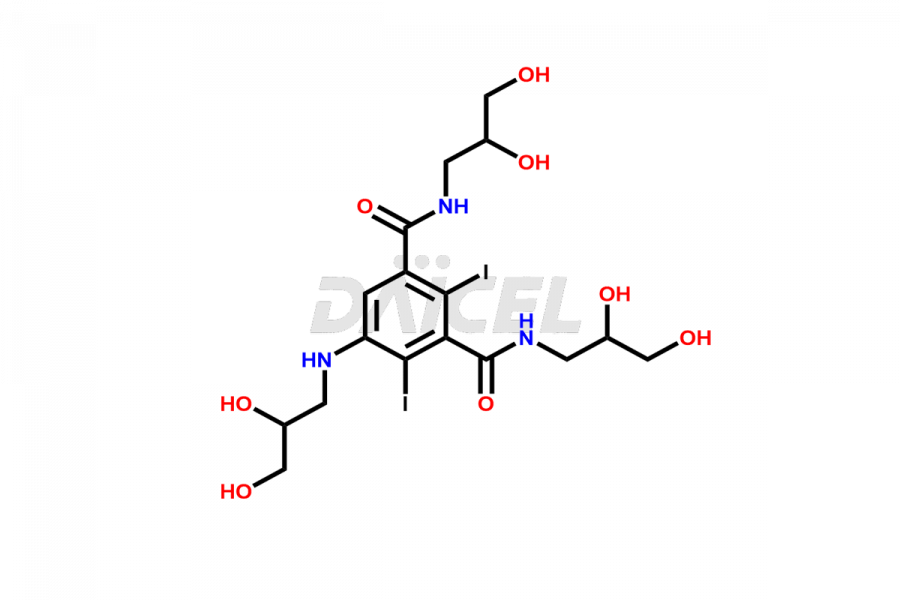
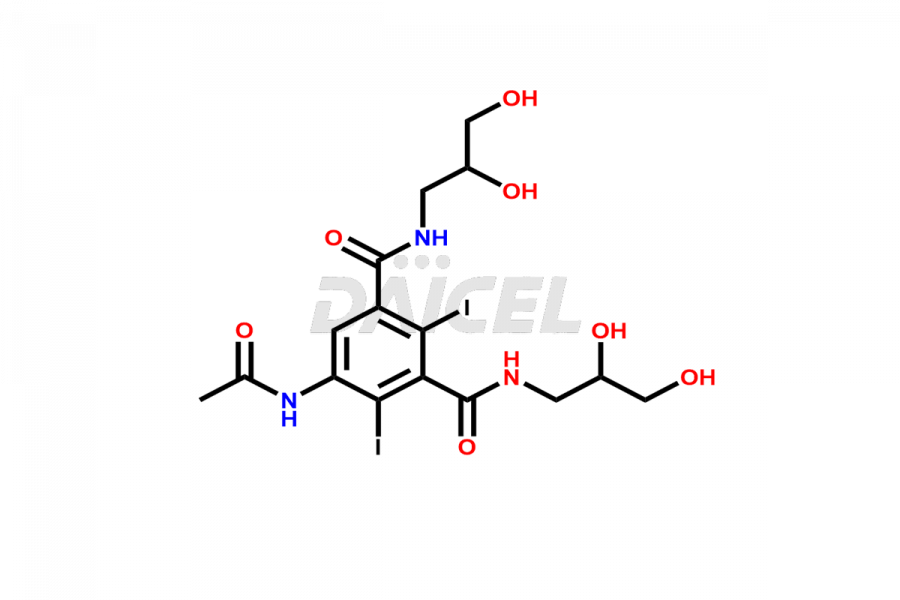
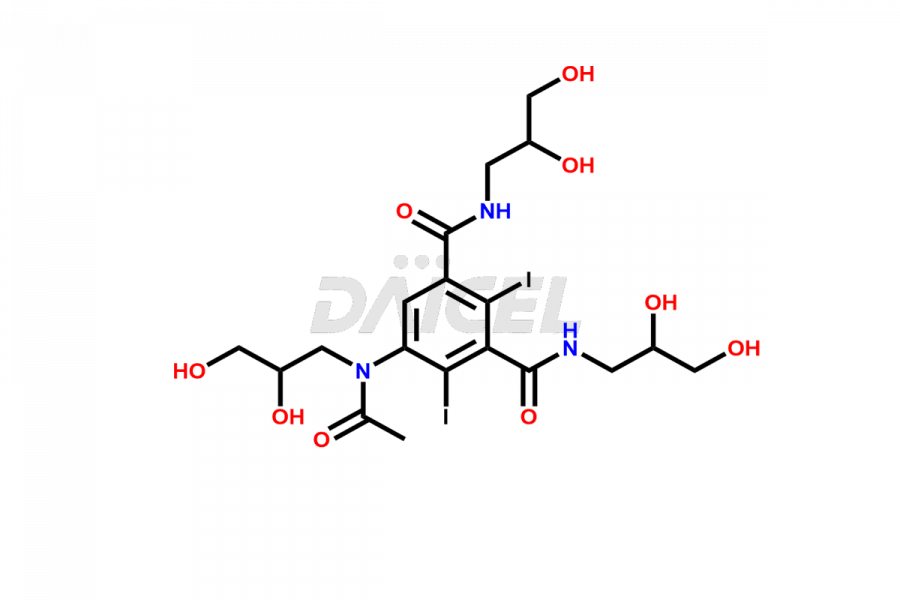
Iohexol Impurities and Iohexol
Daicel Pharma synthesizes top-notch impurity standards for Iohexol, an active pharmaceutical ingredient. We offer crucial impurity standards such as EP IOHEXOL IMPURITY F/ Iohexol impurity F, EP IOHEXOL IMPURITY M/ Iohexol impurity M, IOHEXOL EP IMPURITY G/ Iohexol Impurity G, IOHEXOL EP IMPURITY H/ Iohexol Impurity H, and IOHEXOL EP IMPURITY I/ Iohexol Impurity I, which play a vital role in evaluating the purity, and safety of Iohexol. Daicel Pharma also provides a custom synthesis of Iohexol impurity standards to meet specific client needs and worldwide delivery options.
In various radiographic procedures, Iohexol [CAS: 66108-95-0] is an effective non-ionic water-soluble X-ray contrast medium used in radiographic procedures. It is for myelography, arthrography, nephroangiography, arteriography, and other similar methods. The concentration of Iohexol in the medium varies from 140 to 350 milligrams of iodine per milliliter.
Iohexol: Use and Commercial Availability
Omnipaque 12, Omnipaque 140, Oraltag, etc., are the brands that contain Iohexol. Iohexol is a safe, non-ionic, low osmolar contrast agent with a molecular weight of 821 Da. Its eliminated by kidneys through filtration. It does not undergo metabolism, secretion, or reabsorption. Iohexol is an excellent marker of glomerular filtration rate (GFR) and serves as a suitable alternative to radiotracers, which require special handling and disposal.
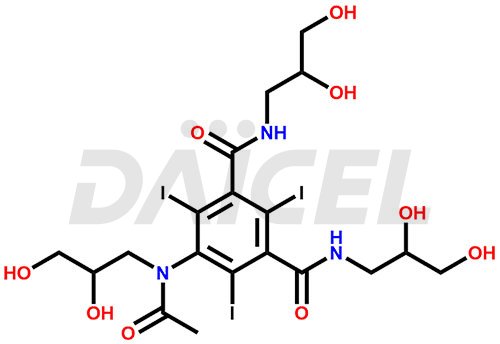
Iohexol Structure and Mechanism of Action 
The chemical name of Iohexol is 5-[Acetyl(2,3-dihydroxypropyl)amino]-N1, N3-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide. Its chemical formula is C19H26I3N3O9, and its molecular weight is approximately 821.1 g/mol.
Iohexol, with its high iodine content, enhances imaging during CT
examinations.
Iohexol Impurities and Synthesis
The control and analysis of impurities in Iohexol are vital to ensure its quality and safety as a contrast agent. They can originate from various sources, including the synthetic process1 and storage conditions. It is essential to monitor and control impurities at every stage of preparation. Analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS) help in impurity profiling and quantification. Stringent quality control measures can meet regulatory standards and ensure the purity and safety of Iohexol. Regular testing and monitoring help minimize impurities and maintain the desired drug quality.
Daicel Pharma, in adherence to cGMP standards, operates an analytical facility where we prepare Iohexol impurity standards like EP IOHEXOL IMPURITY F/ Iohexol impurity F, EP IOHEXOL IMPURITY M/ Iohexol impurity M, IOHEXOL EP IMPURITY G/ Iohexol Impurity G, IOHEXOL EP IMPURITY H/ Iohexol Impurity H, and IOHEXOL EP IMPURITY I/ Iohexol Impurity I. We offer a comprehensive Certificate of Analysis (CoA) for these impurity standards, providing a detailed characterization report. The CoA includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Iohexol impurity standards or degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Nordal, Vegard; Holtermann, Hugo, Triiodoisophthalic Acid Amides, NYEGAARD & CO. A/S’s GB1548594A, July18, 1979
- Edelson, Jerome; Palace, Gerard; Park, George, High-performance liquid chromatographic determination of iohexol in plasma, urine and feces, Journal of Chromatography, Biomedical Applications, Volume: 274, Pages: 428-33, 1983
Frequently Asked Questions
What steps help remove or reduce Iohexol impurities?
Purification techniques, such as recrystallization, chromatography, or filtration, may be employed during the preparation process of Iohexol to remove or reduce impurities to acceptable levels.
Are there any specific stability requirements for impurity control in Iohexol?
Yes, stability studies help assess the long-term behavior of impurities in Iohexol under various storage conditions. It helps establish appropriate storage recommendations and shelf-life for the drug product.
Which solvent helps in analyzing Iohexol impurities?
Water is commonly used as a solvent when analyzing many impurities in Iohexol.
How should Iohexol impurities be stored in terms of temperature?
The recommendation is to store Iohexol impurities at room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.