Indomethacin
General Information
Indomethacin Impurities and Indomethacin
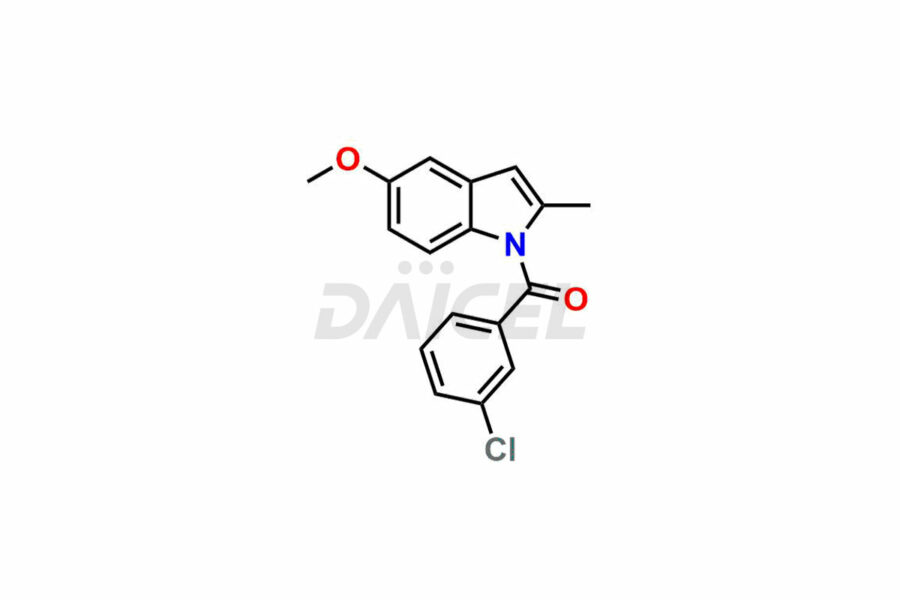
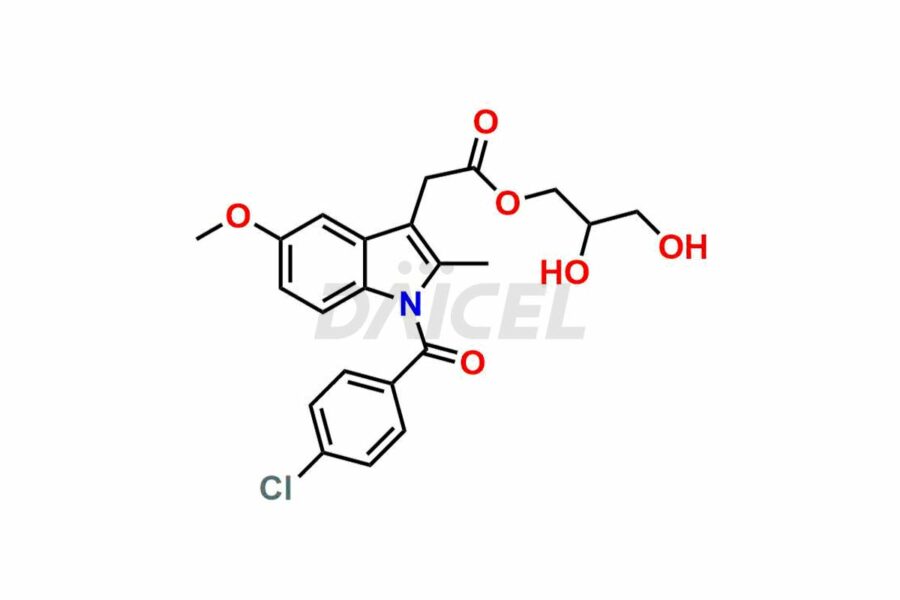
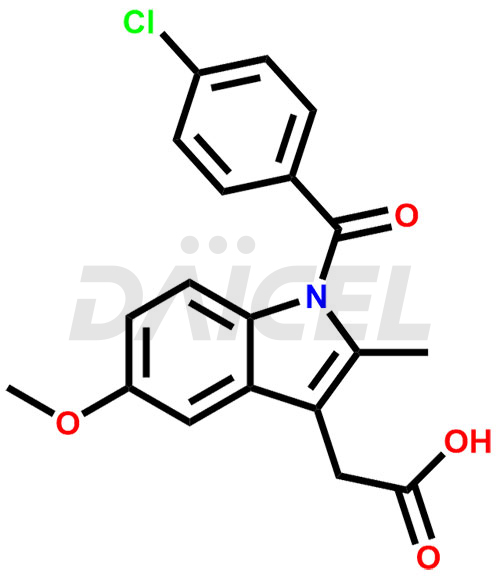
Daicel Pharma synthesizes top-notch impurity standards for Indomethacin, an active pharmaceutical ingredient. We offer crucial impurity standards such as 1-(3-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indole, Indomethacin alpha monoglyceride, and Indomethacin EP Impurity-E, which play a vital role in evaluating the purity, and safety of Indomethacin. Daicel Pharma also provides a custom synthesis of Indomethacin impurity standards to meet specific client needs and worldwide delivery options.
Indomethacin [CAS: 53-86-1] is a synthetic non-steroidal indole derivative with chemopreventive properties and anti-inflammatory activity. It acts as a non-steroidal anti-inflammatory agent (NSAIA) and exhibits analgesic and antipyretic effects. By inhibiting cyclooxygenase, which is responsible for the formation of prostaglandins and other autacoids, it functions as an NSAID. Furthermore, Indomethacin also hampers the motility of polymorphonuclear leukocytes.
Indomethacin: Use and Commercial Availability
Indocin, Indocin SR, Indo-Lemmon, and Tivorbex are the brand names of drugs containing Indomethacin. Indomethacin is a US FDA-approved non-steroidal anti-inflammatory (NSAID) medication. It inhibits the synthesis of prostaglandins necessary for inflammation, pain, and fever. The drug is for acute pain, rheumatoid arthritis, ankylosing spondylitis, osteoarthritis, bursitis, gouty arthritis, and patent ductus arteriosus.
Indomethacin Structure and Mechanism of Action 
The chemical name of Indomethacin is 1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetic acid. Its chemical formula is C19H16ClNO4, and its molecular weight is approximately 357.8 g/mol.
Indomethacin inhibits cyclooxygenase (COX-1 and COX-2) and prevents prostaglandin synthesis.
Indomethacin Impurities and Synthesis
Controlling the synthesis1, analysis, and impurities of Indomethacin is crucial to ensure the quality and safety of the medication. During synthesis and storage, impurities can arise and potentially impact the efficacy and stability of Indomethacin. Different synthetic routes help synthesize Indomethacin and closely monitor and control impurity levels at each step. Analytical techniques such as high-performance liquid chromatography (HPLC), liquid chromatography (LC), and mass spectrometry (MS) help in the analysis and quantification of impurities. Stringent quality control measures can adhere to regulatory requirements and maintain the purity and safety of Indomethacin.
Daicel Pharma, in adherence to cGMP standards, operates an analytical facility where we prepare Indomethacin impurity standards like 1-(3-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indole, Indomethacin alpha monoglyceride, and Indomethacin EP Impurity-E. We offer a comprehensive Certificate of Analysis (CoA) for these impurity standards, providing a detailed characterization report. The CoA includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Indomethacin impurity standards or degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Hisao Yamamoto, Nishinomiya-shi, Masaru Nakao, Process For The Preparation Of 3-Indolyl Aliphatic Acid Derivatives, Sumitomo Chemical Co., Ltd., US3629284A, December 21, 1971
- Skellern, G. G.; Salole, E. G., High-speed liquid chromatographic analysis of indomethacin in plasma, Journal of Chromatography, Volume: 114, Issue: 2, Pages: 483-5, 1975
Frequently Asked Questions
What are the challenges in controlling Indomethacin impurities?
The challenges in controlling Indomethacin impurities include complex synthetic processes, interactions between impurities and API, and sensitive analytical techniques to detect and quantify the trace levels.
Can Indomethacin impurities change over time?
Yes, impurity profiles in Indomethacin can change over time due to various factors such as storage conditions, degradation, or interaction with packaging materials. Regular monitoring is essential to ensure ongoing control.
Which solvent helps in analyzing Indomethacin impurities?
Acetonitrile or Chloroform are commonly used as solvents when analyzing many impurities in Indomethacin.
How should Indomethacin impurities be stored in terms of temperature?
The recommendation is to store Indomethacin impurities at room temperature, within 2-8 ⁰C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.